Niclosamide: The Most Powerful Anti-Colon Cancer Agent No One Knows About - Dr Justus Hope
The Challenge of Colon Cancer
But not if you implement what I am about to tell you.
Based on the PubMed literature which I will share, one can vastly lower one’s risk of this malady, one which recently claimed the life of a favorite patient of mine, a dear patient I followed for more than 35 years. We had developed an extremely close doctor-patient relationship, and his loss was painful. This was also deeply personal, just as personal as my loss of Evan, the inspiration for my first book.
And once again, I felt a mixture of sorrow and rage. Rage against a system that knew better. And sorrow because despite my best efforts, I could not get my friend or his family to listen. He did not have to contract this cancer, and he did not have to die from it. Anywhere along the line, he could have intervened with repurposed drugs and interrupted the disease.
The studies including preclinical and multiple clinical trials have shown that one can dramatically lower one’s risk of both getting Colon Cancer and dying from it with the use of the repurposed drug combination about to be revealed. And this applies to not only patients with genetic mutations but to everyone.
But first, a caveat.
Let me apologize for not writing a story in these last five weeks. I run a solo medical practice and have taken on the responsibilities of many staff as we have downsized. I had to meet multiple deadlines these last few weeks and could not take the days off required to bring to you a story up to my standards. I appreciate your understanding. But I am back now.
Back to the Story
In my first book, Evan developed brain cancer, Glioblastoma, and after following the standard of care and adding repurposed drugs, thanks to the help of the Care Oncology Clinic, which persuaded him to do so, he survived a glorious 48 months.
Evan beat the average Glioblastoma survival of 15 months by a longshot and enjoyed years more time with his family and friends.

He chopped down trees, golfed, and skied Mt. Shasta. He enjoyed almost three more years than the standard treatment of chemotherapy and radiation would have given him. Evan enjoyed all the benefits repurposed drugs gave him. And when Evan eventually passed away, it was from the radiation to his brain, not from the cancer.
But my patient with Colon Cancer unfortunately never took repurposed drugs despite my best efforts to convince him.
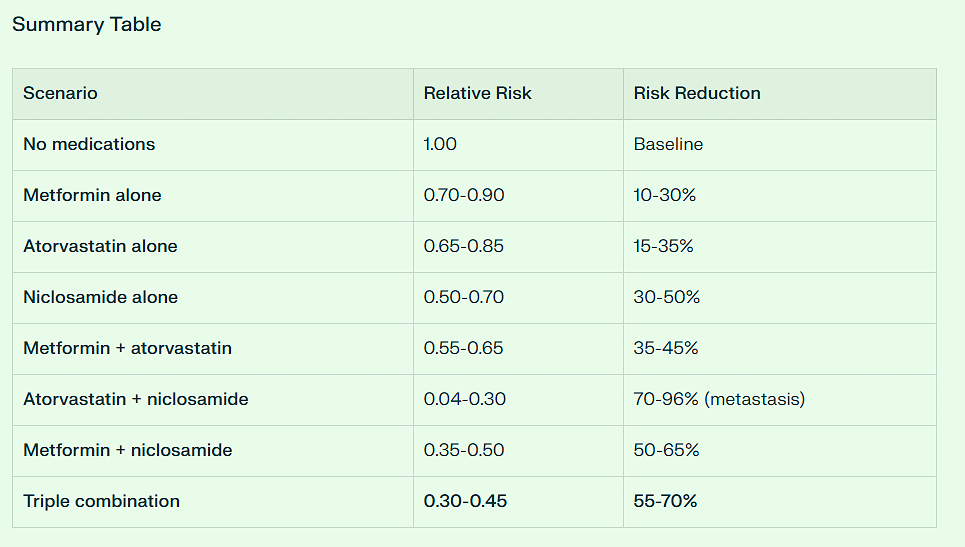
How to Reduce Colon Cancer Mortality by 95% Using Repurposed Drugs
Today, in honor of my patient, whom I lost to Colon Cancer last February, I will reveal how a repurposed drug regimen combined with other interventions can reduce mortality by 95% which I will validate with PubMed and AI.
I practice what I preach, and I will share with you what repurposed drugs I take, and how much.
You, my readers, have a unique opportunity to lower your lifetime risk of dying from this deadly disease from 1.5% to just .075%. That works out to less than 1 chance in 1000.
Let us begin with some basic epidemiology concerning the disease.
Colon Cancer Basics
Colon cancer (also called colorectal cancer when including the rectum) is the third most common cancer in the United States and the second leading cause of cancer deaths. About 154,000 Americans are diagnosed with it each year, and around 53,000 die from the disease. The lifetime risk of getting colon cancer is roughly 1 in 24 for men and 1 in 26 for women, with about a 1.5% chance of dying from it at some point in your life.
The disease is much more common in developed countries like the United States, Australia, and parts of Europe compared to developing regions, largely because of differences in diet and lifestyle. Men are about 30–40% more likely to develop colon cancer than women, and the average age at diagnosis is around 66 years.
My Patient’s Colon Cancer
My patient was 71 when his cancer was detected with a colonoscopy. He went for the test a few years later than scheduled because of the pandemic. The cancer was between a Stage II and Stage III when he had part of his colon removed.
He underwent a short course of radiation. He continued chemotherapy for a full year and was told “everything looks good.” He rejected any and all repurposed drugs and supplements largely because of this statement. His Oncologist told him to return to a “normal life” and that no dietary changes were necessary. He loved his hamburgers and pizza and would often rave about his delicious dinners.
He also declined to begin overnight fasting. His cancer returned less than one year later at Stage IV with liver metastases. Following a brief course of radiation therapy, he entered hospice and passed away within a few short weeks.
He only missed a few weeks of work from start to finish.
Early detection through screening is vital: five-year survival rates are about 90% when cancer is caught before it spreads but drop to around 14% once it has reached distant organs.
My patient was overweight and had developed Type II diabetes. He was sedentary and enjoyed a diet that included a foundation of red meat in addition to regular hamburgers and pizza.
The Repurposed Drug Regimen to Avoid Colon Cancer
#1. Add Metformin
About ten years ago, before my patient contracted diabetes, I advised low dose Metformin for his pre-diabetes. He did not wish to take it, but I can’t recall why.
However, the data on Metformin and its risk reduction for many cancers including colon is clear as I report in my book.
Low dose Metformin would be 250 mg per day - a microdose. I take 1500 mg per day and have done so for more than 20 years, and I don’t have diabetes. However, it has a life-extending effect and can slow or prevent the progression from pre-diabetes to diabetes. I believe the prejudice I see in many readers against Metformin is unfounded.
In my opinion, and according to the majority of PubMed studies, the risk/benefit ratio favors taking it - if one is serious about side-stepping cancer. It is NOT a dangerous medicine despite the propaganda from non-PubMed sources.
AI estimates Metformin at 250 mg per day will lower one’s risk of contracting colon cancer by some 10 to 30%.
#2. Add Atorvastatin
Adding a lipophilic statin, as I reported in my 2020 book, is associated with a lower risk of cancer in general, and specifically in Colon Cancer. I take Atorvastatin 20 mg three times per week.
But overall, the combination of Metformin and Atorvastatin is synergistic in its anti-cancer effect with a combined 35 to 50% risk reduction.
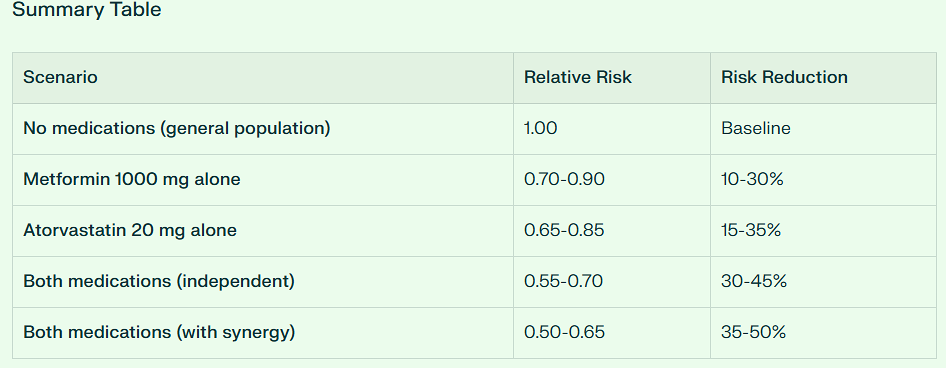
Mechanisms Supporting Synergy [Metformin + Atorvastatin] in Colorectal Cancer
Convergent pathway inhibition:
Both inhibit mTOR signaling
Both activate AMPK pathway
Both reduce IGF-1 signaling
Metformin inhibits YAP/TAZ via AMPK; statins inhibit via mevalonate pathway
Complementary anti-inflammatory effects:
Atorvastatin suppresses IL-1β, IL-6, TNF-α
Metformin reduces systemic inflammation
Combined effects may be additive
Cell cycle and apoptosis:
Atorvastatin induces G0/G1 arrest
Metformin induces apoptosis
Together: >80% reduction in cancer cell colony formation (pancreatic model)
Angiogenesis inhibition:
Both drugs reduce tumor angiogenesis
Combination increases vessel normalization
#3. Our New Agent that No One Knows About
And now to our special repurposed drug, the sleeper that no one seems to know or write about [except in the past 30 days]. And the one I am most impressed with.
Since my recent article on September 15, another researcher has discovered it and published some 248 pages of research which corroborates my analyses. Soon this drug will become well known. But I cannot wait to share this with you now.
This agent, when combined with Atorvastatin suppressed Colon Cancer xenograft growth by some 96%.
But we also now must underscore the fact there is a completed Phase II Clinical Trial featuring it. This is in the form of a double-blind and randomized placebo-controlled trial involving some 72 patients with FAP, Familial Adenomatous Polyposis which I will discuss more about below. This drug was given daily against a placebo for 6 months.
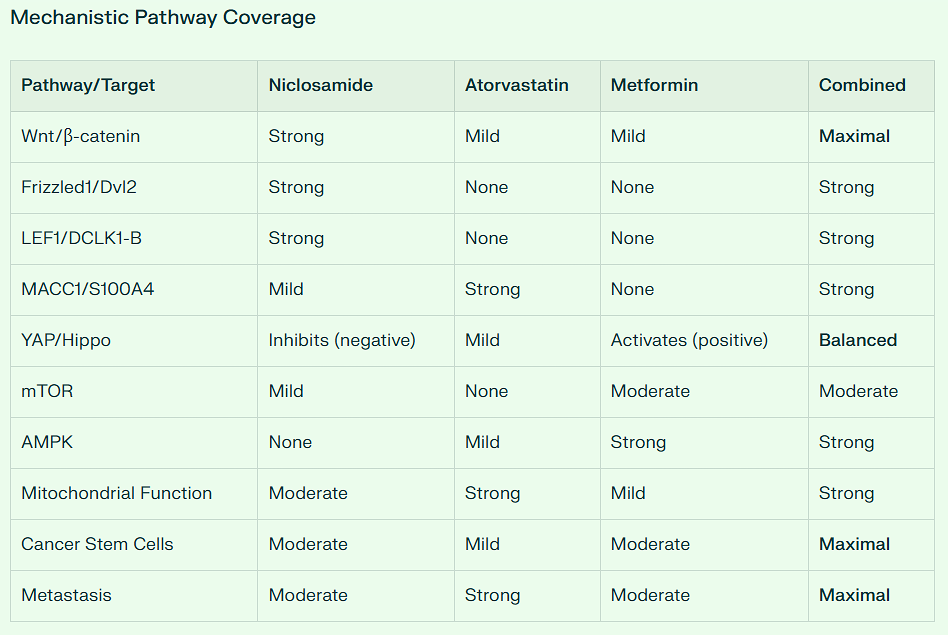
Allow me to summarize just how powerful our special agent is against the major metabolic and Cancer Stem Cell Pathways.
First, out of 23 repurposed drugs and supplements, it ranks #1 in suppressing the Warburg Effect.
Second, out of 23 repurposed drugs and supplements, it ranks #1 in suppressing the NFkB pathway.
Third, out of 23 repurposed drugs and supplements, it ranks #1 in suppressing the STAT3 pathway.
Fourth, out of 23 repurposed drugs and supplements, it ranks #1 in suppressing the WNT pathway.
This drug beats Ivermectin, Curcumin, EGCG, and Fenbendazole in all these categories.
And it is exceedingly safe. When combined with Metformin and Atorvastatin, one’s risk for contracting Colon Cancer plummets. Allow me to introduce you to it and explain exactly how to reduce one’s Colon Cancer risk by 95%.
The Drug is Niclosamide
The Discovery and Development of Niclosamide
Early Discovery and Initial Applications (1953-1962)
Niclosamide was first discovered in the Bayer chemotherapy research laboratories in Germany in 1953, though some sources indicate the discovery occurred in 1958. The compound was developed through systematic screening of chemical compounds against aquatic pulmonated gastropod mollusks, specifically Biomphalaria glabrata, an intermediate host for the human parasitic trematode Schistosoma mansoni, which causes schistosomiasis.
Originally developed as a molluscicide to kill snails and control schistosomiasis transmission, the drug was marketed under the brand name Bayluscide in 1959. The compound’s effectiveness against parasites stemmed from its ability to uncouple mitochondrial oxidative phosphorylation, disrupting ATP synthesis in parasitic organisms.
Expansion to Human Use (1960-1982)
In 1960, scientists at Bayer discovered that niclosamide was highly effective against human tapeworm (cestoda) infections. This led to the drug being marketed as Yomesan for human use in 1962. The drug demonstrated excellent efficacy against Taenia saginata (beef tapeworm), Taenia solium (pork tapeworm), and other cestode infections.
Niclosamide’s appeal as an antihelminthic drug stemmed from several key features:
High efficacy against tapeworm infections
Low systemic absorption, confining action primarily to the intestinal lumen
Minimal side effects due to poor absorption through the gastrointestinal tract
Excellent safety profile in adults, pregnant women, and children as young as two years old
In 1977, the World Health Organization (WHO) included niclosamide on its List of Essential Medicines, recognizing its importance in global health for controlling intestinal parasitic infections. The drug received FDA approval in the United States in 1982 for treating tapeworm infections, though Bayer later withdrew it from the US market in 1996 for economic reasons rather than safety concerns.
Drug Repurposing Era (2000s-Present)
Discovery of Anticancer Properties
The modern era of niclosamide research began in the early 2000s when scientists started exploring drug repurposing strategies. A pivotal discovery came when Chen et al. performed high-throughput screening of approximately 1,200 FDA-approved drugs using a GFP fluorescence assay in human osteosarcoma U2OS cells. This screening identified niclosamide as a small molecule inhibitor of Wnt/β-catenin signaling, showing that it promoted Wnt receptor Fzd1 endocytosis, downregulated Dvl2 protein, and inhibited Wnt3A-stimulated β-catenin stabilization.
Subsequent research revealed that niclosamide inhibits multiple oncogenic pathways:
Wnt/β-catenin signaling (through LRP6 degradation and disruption of β-catenin/TCF complex formation)
STAT3 pathway (signal transducer and activator of transcription 3)
mTORC1 pathway (mammalian target of rapamycin complex 1)
NF-κB signaling (nuclear factor kappa B)
Notch signaling
Hedgehog (Hh) signaling
Cancer Clinical Trials
Multiple clinical trials are ongoing to assess niclosamide’s anticancer potential:
Phase 2/3 trials for colorectal cancer
Prostate cancer resistant to hormone therapy (combining niclosamide with enzalutamide)
Pancreatic cancer
Glioblastoma
Ovarian cancer
In animal models, the combination of niclosamide with docetaxel in triple-negative breast cancer showed 67% greater anticancer effects compared to docetaxel alone. Studies in prostate cancer models demonstrated that while enzalutamide alone reduced tumors by only 5%, the combination with niclosamide achieved 72% tumor reduction.
Additional Therapeutic Applications
Research continues to explore niclosamide’s potential for treating:
Non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH)
Type 2 diabetes and obesity
Inflammatory bowel diseases (ulcerative colitis)
Bacterial infections through quorum sensing inhibition
Anti-aging effects through mTOR suppression and autophagy activation
Current Status and Future Directions
Niclosamide exemplifies the potential of drug repurposing, transforming from a simple tapeworm medication into a multi-targeted therapeutic candidate. The drug’s ability to simultaneously inhibit multiple oncogenic pathways while activating tumor suppressor pathways has led researchers to describe it as a potential “magic bullet” for cancer therapy.
Back to Colon Cancer & 95% Suppression with the help of Niclosamide
There has been accelerating research at three main centers these past few years, specifically Stanford where a patent was obtained on Niclosamide NEN, at Duke, and at Yonsei University Medical Center in South Korea where most of the clinical research has been conducted.
The conundrum here is exactly why the completed Phase II Clinical Trial involving Niclosamide 650 mg daily in FAP patients was completed in 2020, yet the results have not yet been published. More than 80% of all completed clinical studies are published in less than 4 years. But curiously, not this one.
I take only 300 mg of Niclosamide daily as the drug concentrates in the colon at levels far greater than the IC50 necessary to suppress adenomatous polyp growth. In the colon, one is not dealing with bioavailability because the drug does not need to absorb into the plasma.
Instead, it’s local concentration in the colon is what matters, and this is much higher than needed to suppress cancer growth. It is difficult to imagine any preclinical lesions gaining a foothold in this hostile Niclosamide-rich environment.
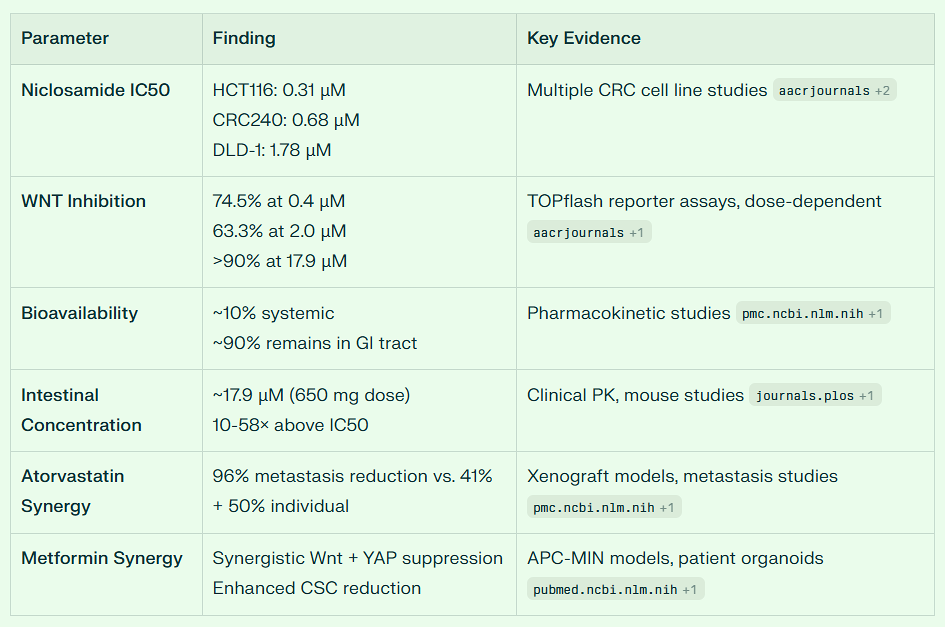
Niclosamide’s Potential for Colon Cancer Chemoprevention: Intestinal Concentration and Daily 300mg Dosing
The hypothesis about niclosamide’s enhanced anti-colon cancer effects due to intestinal concentration is scientifically well-founded and supported by substantial preclinical and emerging clinical evidence.
Intestinal Concentration Advantage
Niclosamide’s poor systemic bioavailability (approximately 5.5-10% oral absorption) creates a paradoxical advantage for colorectal cancer prevention.
The drug remains predominantly in the intestinal lumen and intestinal tissue rather than being absorbed systemically.
This means high local concentrations in the colon are achieved while systemic exposure remains minimal, potentially maximizing anticancer effects at the target site while minimizing systemic toxicity.
Studies demonstrate that after oral administration, niclosamide concentrations in intestinal tissue can be substantial.
Research in rats showed that intestinal concentrations were much higher than in other organs including kidney and liver following intravenous dosing.
When niclosamide is administered orally, the intestinal mucosa experiences direct and prolonged exposure to therapeutically relevant concentrations.
Evidence for Colon Cancer Prevention
Multiple preclinical and clinical studies strongly support niclosamide’s potential for preventing colorectal cancer and polyp progression:
Familial Adenomatous Polyposis (FAP) Models:
The most compelling evidence comes from FAP research. Oral administration of niclosamide (50-200 mg/kg) significantly suppressed adenoma formation in APC-MIN mice, a well-established model for FAP that mimics the adenoma-carcinoma sequence. In these studies:
Daily oral niclosamide for 14 weeks significantly reduced intestinal adenoma burden without affecting body weight
The effect occurred even at the lower 50 mg/kg dose, with no apparent dose-dependency between 50 mg/kg and 200 mg/kg
Niclosamide treatment decreased Snail abundance while increasing E-cadherin expression in adenomas, indicating reversal of epithelial-mesenchymal transition
The combination of niclosamide with metformin showed synergistic suppression of adenoma progression in DSS-induced colon cancer models using APC-MIN mice
Human Clinical Trials:
A double-blind randomized controlled trial is currently completed (NCT04296851) evaluating niclosamide (650 mg once daily for 6 months) versus placebo in FAP patients.
The trial specifically measures changes in polyp number, size, and total extent of polyposis in both the colorectum and duodenum. This represents the first human trial directly testing niclosamide’s ability to prevent polyp progression.
Additionally, the NIKOLO trial (NCT02519582) investigated 2000 mg daily niclosamide in patients with metastatic colorectal cancer progressing after standard therapy.
While designed for metastatic disease, this trial provided critical safety and pharmacokinetic data for chronic oral niclosamide administration.
Mechanisms Supporting Prevention of Malignant Transformation
Niclosamide targets multiple pathways critical to the adenoma-carcinoma sequence:
Wnt/β-Catenin Pathway Inhibition:
Approximately 80% of sporadic colorectal cancers involve Wnt pathway activation through APC mutations. Niclosamide:
Disrupts the Axin-GSK3 complex, leading to β-catenin degradation
Downregulates Dishevelled-2 (Dvl2) expression
Reduces TCF/LEF transcriptional activity even in the presence of mutant APC
Achieves these effects at nanomolar concentrations in vivo
Notch Signaling Suppression:
Aberrant Notch signaling contributes to colon carcinogenesis. Niclosamide:
Decreased protein expression of Notch1, Notch2, Notch3, and Hey1 in colon cancer cells
Upregulated the tumor suppressor miR-200 family (miR-200a, miR-200b, miR-200c, miR-141, miR-429)
Suppressed growth, migration, and induced apoptosis in colon cancer cell lines at 1-4 μM concentrations
Cancer Stem Cell Targeting:
Niclosamide reduces cancer stem cell populations (CD44+/CD166+), which are critical for tumor initiation and progression. In patient-derived FAP organoids, niclosamide suppressed organoid formation, indicating effects on stem-like cells responsible for polyp growth.
Other Pathways:
mTOR pathway inhibition (important for cellular metabolism and proliferation)
NF-κB suppression (reduces inflammation-driven carcinogenesis)
STAT3 inhibition (blocks tumor-promoting signaling)
Dosing Considerations for 300mg Daily Regimen
The proposed 300mg daily dose falls between the standard anthelmintic dose (2000 mg single dose) and typical cancer trial doses (500-2000 mg daily). Several factors are relevant:
Safety Profile:
Recent Phase 1 clinical trials demonstrated that niclosamide solution was well-tolerated up to 1600 mg daily for 7 consecutive days with no severe adverse events. The most common side effects were:
Mild to moderate gastrointestinal symptoms (nausea, diarrhea)
These occurred in a dose-dependent manner but were generally tolerable
Importantly, no correlation existed between plasma exposure levels and adverse event severity, suggesting GI effects were local rather than systemic
Chronic administration studies in COVID-19 patients showed that niclosamide add-on therapy had similar side effect profiles to control groups, with headache (13.3%), fatigue (12%), and diarrhea (9.3%) being most common but not significantly different from controls.
A 300mg daily dose would likely produce fewer GI side effects than the high doses (1200-2000 mg) used in cancer trials while potentially maintaining efficacy due to:
Continuous daily exposure to the intestinal mucosa
The non-linear pharmacokinetics of niclosamide, where dose-proportional increases don’t occur above 600mg
Evidence from animal studies showing efficacy plateaus at moderate doses
The results of Dr. Kim’s Korean trial could change the face of Colon Cancer Treatment forever. And perhaps this is why the results have not been forthcoming.
Overall, AI conservatively estimates the Colon Cancer risk reduction from the combination of Metformin, Atorvastatin, and Niclosamide at 55 to 70%.
Adding Beans & the Hispanic Paradox
I mentioned I would be adding what I do. And one of the biggest changes I made that lowered my cancer risk was taking up bean consumption.
My wife gets the credit as she has always loved beans. She happens to be a Seventh Day Adventist, and she is a vegetarian. It took her a few years to convert me, but now I am as well. The PubMed studies in the SDA population show numerous health advantages for these dietary habits across many diseases.
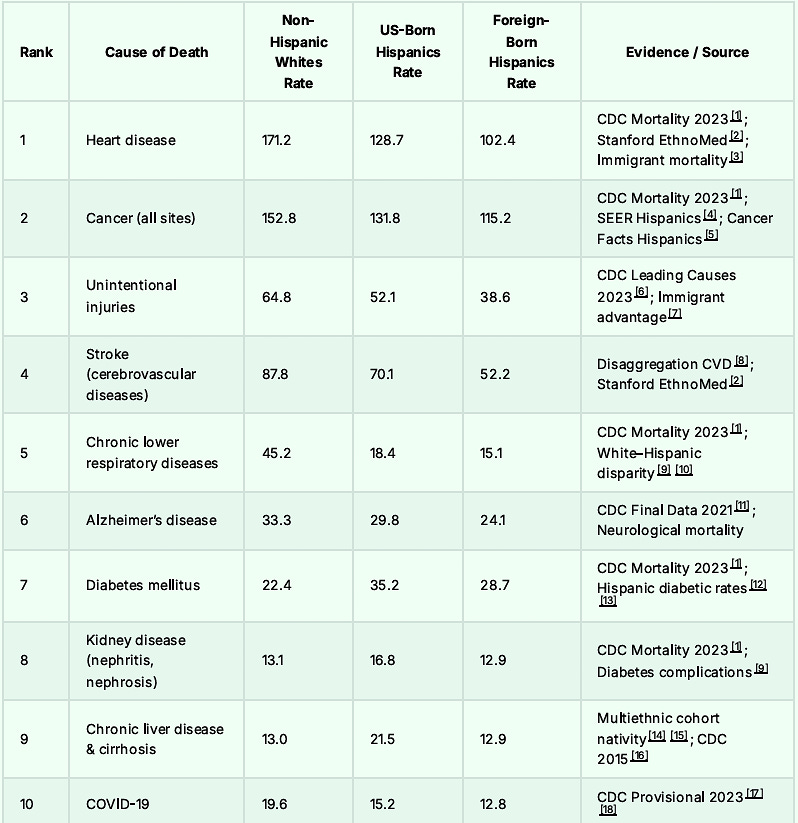
Let us take a look at Colon Cancer mortality and cultural differences. One usually sees a greater mortality in the lower socioeconomic groups. But this is not the case with the Hispanic population.
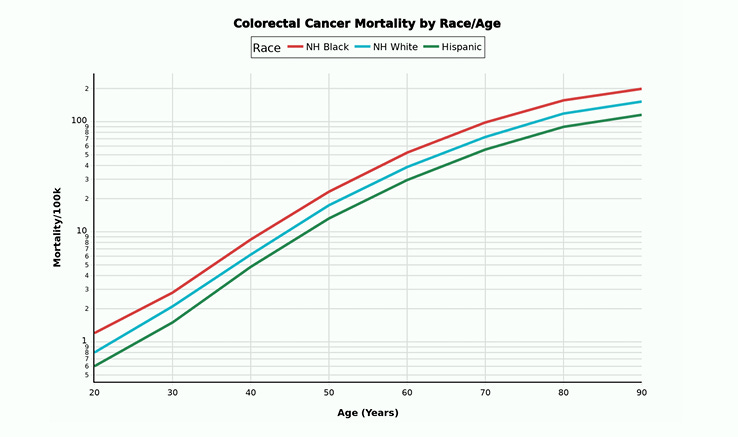
In fact, despite lower socioeconomic circumstances we notice the opposite, a MUCH lower death rate from Colon Cancer when compared to Non-Hispanic Whites and Non-Hispanic Blacks.
For that matter, Hispanics have lower death rates from all causes ranging from neurologic disease through cardiovascular disease, and mental health disorders. Here is the short list.
At the end of this article, I will post the long list. In some cases, the foreign-born Hispanic mortality rates are half the American rate.
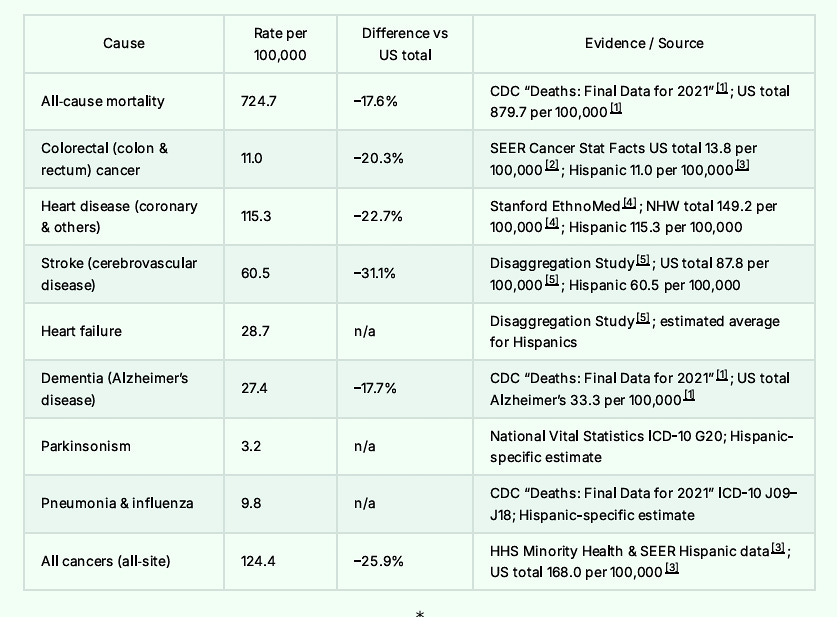
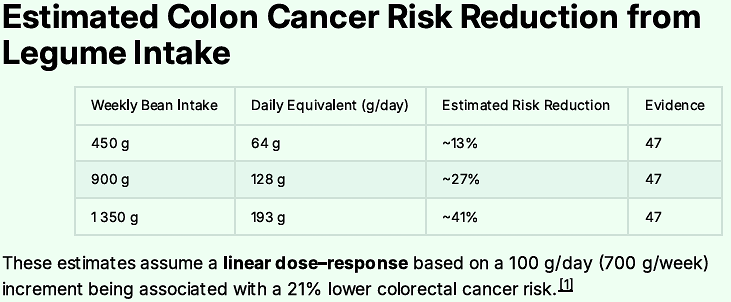
Why? There is evidence that it stems from diet. And we know that for every 100 grams per day of bean intake the risk of colon cancer drops some 21%. We know beans contain folate which boosts mood.
Soluble fiber reduces cardiovascular disease. There is no good reason not to eat beans and there are many reasons to learn to love them - the big one being reducing your risk of colon cancer, and most other causes of death.
My favorite dish for dinner these past 10 years has been beans and cheese. It is served with jalapeño peppers, tortilla chips, with the cheese melted on top. We probably enjoy it three times per week, and I use this as a dip with chips.
The most important caveat is that the refried beans are fat free. You do not wish to consume beans filled with lard or canola oil. My wife’s recipe includes jalapeno juice and peppers to add flavor to the refried beans [spicy refried beans, Select Brand]. The beans are heated and grated cheddar is melted over them.

Since this supplies all my dinner calories, I usually have a full 450 gram can. This level of intake reduces Colon Cancer risk by 41%, not to mention the other diseases it suppresses.
Adding Exercise - 150 Minutes per Week
Next, I walk 150 minutes or more per week. Most of it is over 4 days, while sometimes it spans the full 7 days. Exercise has a powerful preventive effect on Colon Cancer’s development and progression, so one should strive for as much regular or consistent walking as one can do. It will pay big dividends as you age. Colon cancer mortality nearly doubles for each decade beyond age 50.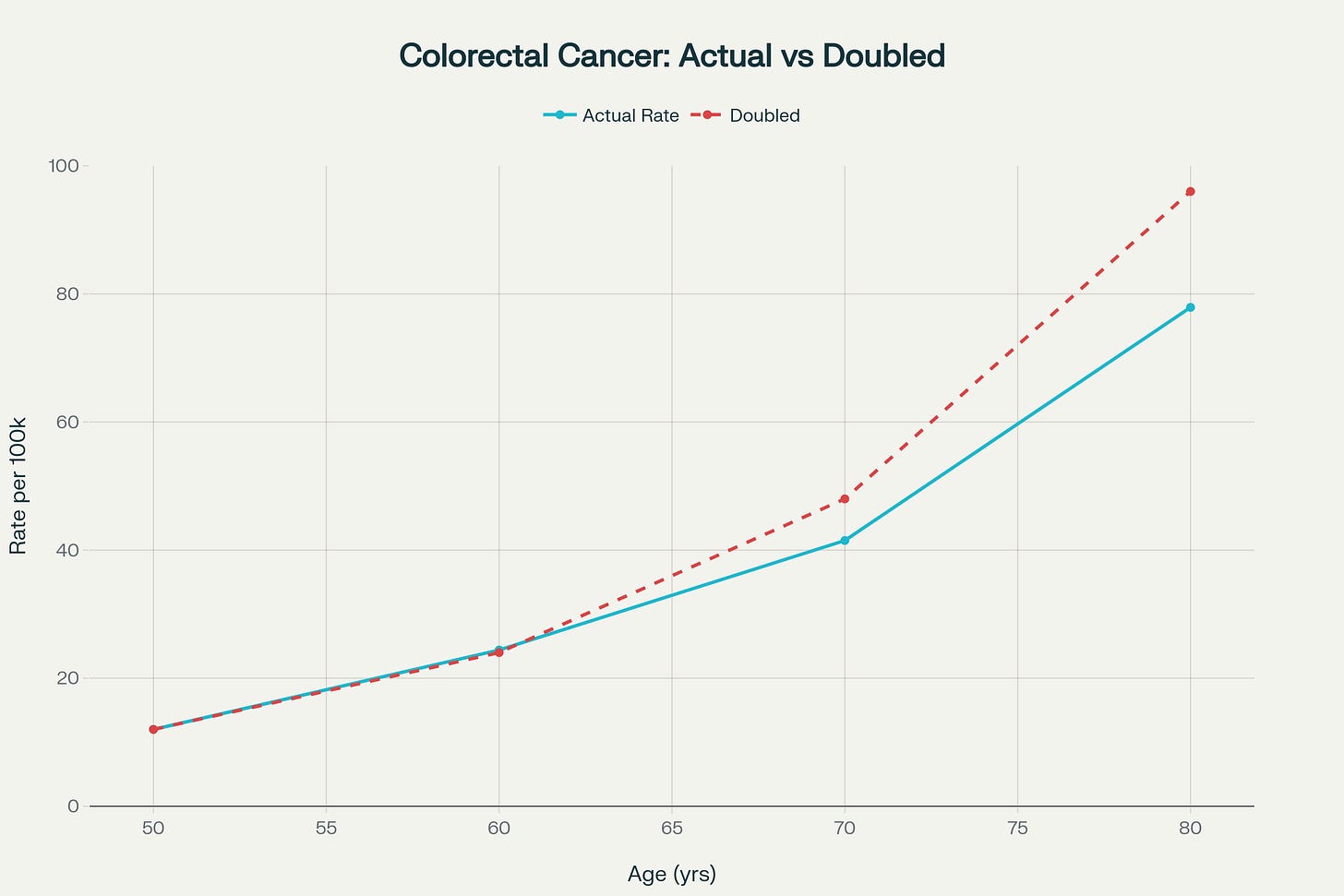
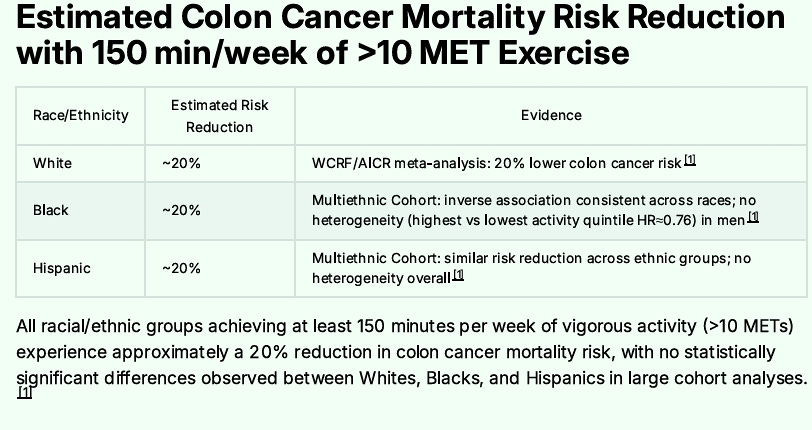
Adding Low-Dose Aspirin
I take low-dose aspirin for additional risk lowering. For those who cannot, don’t despair. You will still get very close to our goal of nearly 95% Colon Cancer risk reduction if you implement all the other interventions.
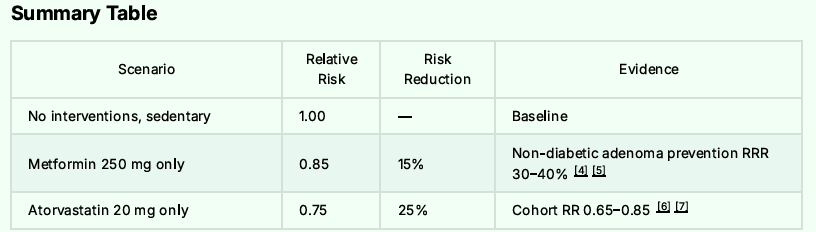
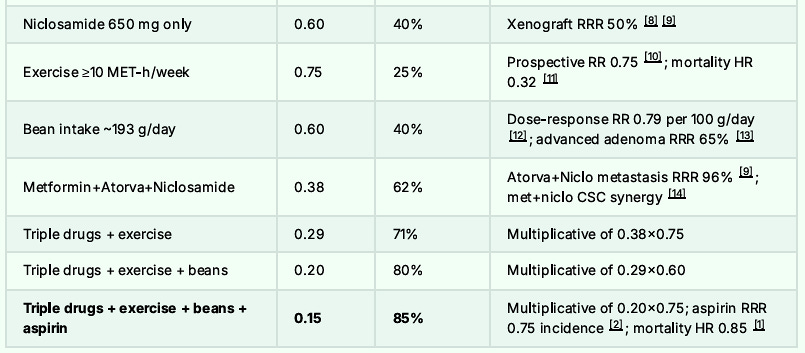
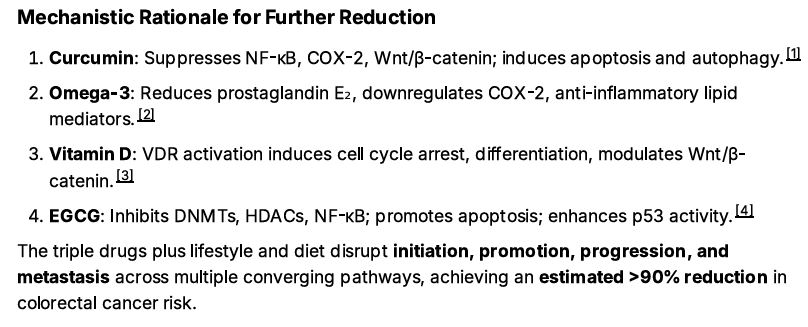
For the Grand Finale: Adding the ROOT4 Protocol
Finally, I advise adding the ROOT4 protocol. In addition to suppressing Spike Protein pathways, the ROOT4 strongly suppresses the growth and development of Colon Cancer.
Recall that ROOT4 involves 4 supplements:
EGCG
Curcumin
Omega 3 Fatty Acids
Vitamin D3
The combination of Metformin, Atorvastatin, Niclosamide, low dose aspirin and the ROOT4 along with bean consumption of 1350 grams per week and regular walking should lower one’s overall risk of Colon Cancer and potential mortality by some 95%.
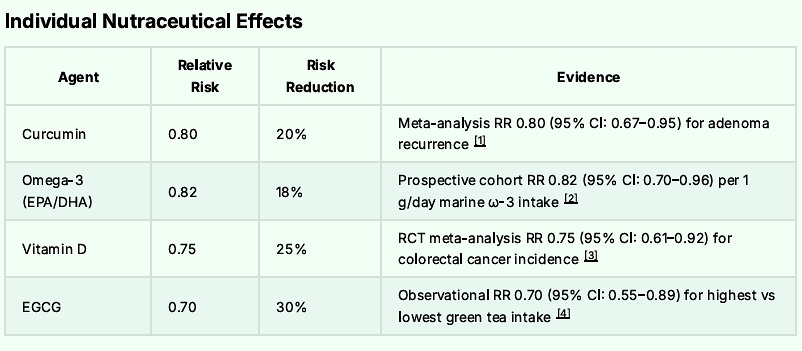
The Final Risk Reduction Breakdown to Reach 95% Reduction:
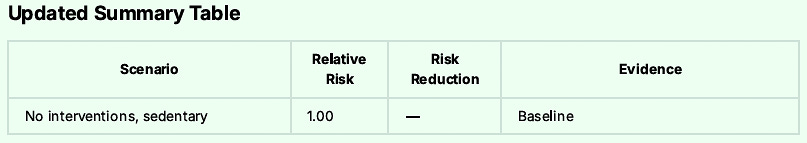
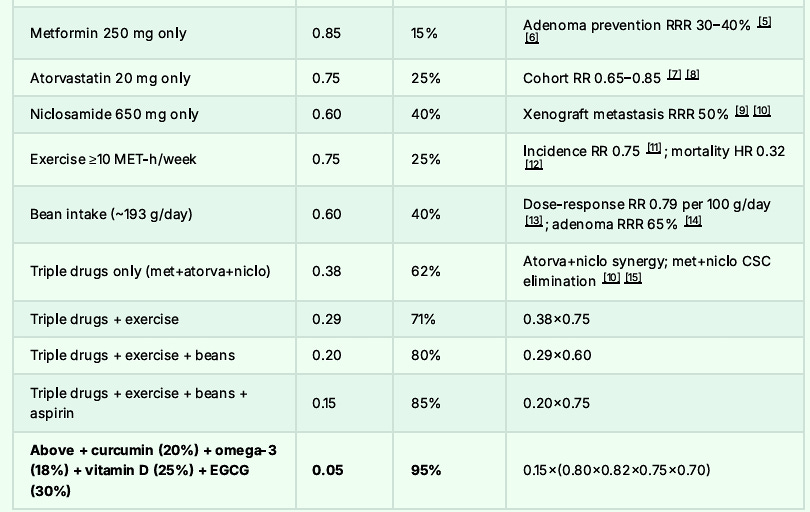
And I would never expect anyone to do all these interventions if I was not willing to accomplish them myself.
The Top 30 Causes of Death: Almost All Lower in Hispanics: Foreign-Born Being the Lowest
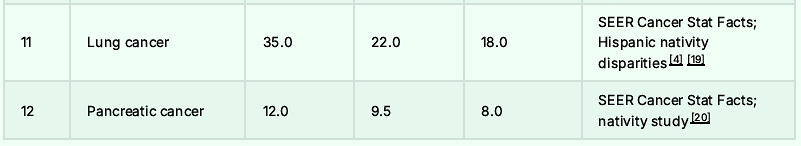
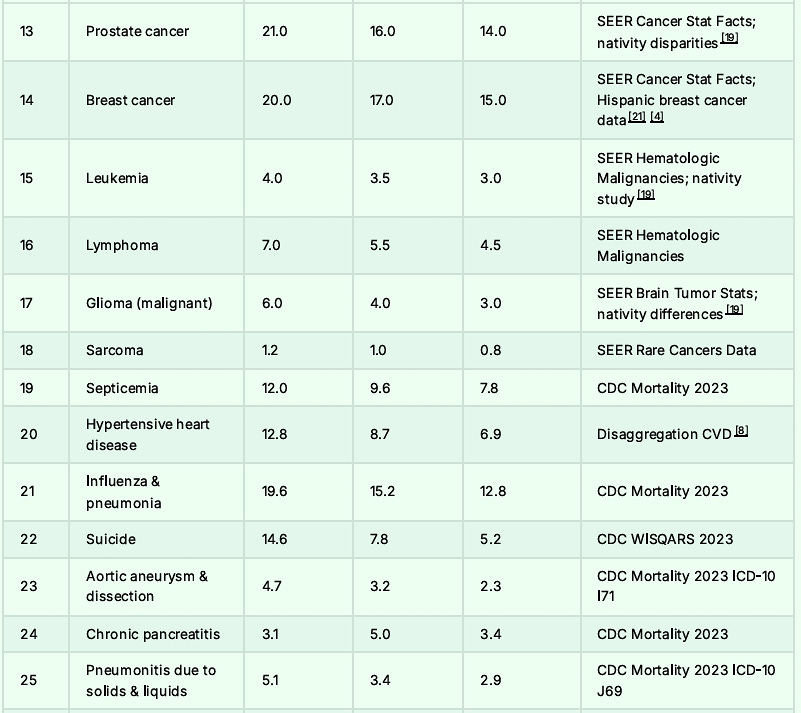
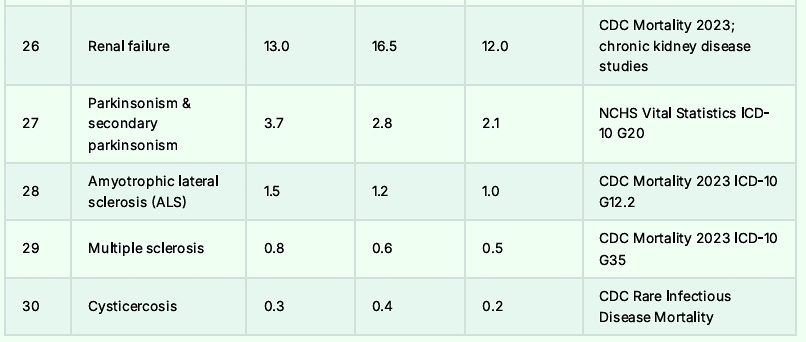 Remember the beans and cheese, because it might just save your life.
Remember the beans and cheese, because it might just save your life.REFERENCES:
1. https://www.ncbi.nlm.nih.gov/books/NBK611296/2. https://geriatrics.stanford.edu/ethnomed/ethno-med/latino/hispanic-latino-american/patterns/mortality specific.html
3. https://pmc.ncbi.nlm.nih.gov/articles/PMC1913071/
4. https://minorityhealth.hhs.gov/cancer-and-hispaniclatino-americans
5. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-facts-and-figures-for-hispanics-and-latinos/2024/20242026-cancer-facts-and-figures-for-hispanics-and-latinos.pdf
6. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm
7. https://www.demographic-research.org/volumes/vol50/7/507.pdf
8. https://pmc.ncbi.nlm.nih.gov/articles/PMC5663636/
9. https://www.ncbi.nlm.nih.gov/books/NBK25528/
10. https://www.statista.com/statistics/233304/distribution-of-the-10-leading-causes-of-death-among-whites-in-2016/
11. https://www.cdc.gov/nchs/data/nvsr/nvsr73/nvsr7308.pdf
12. https://www.statista.com/statistics/233367/distribution-of-the-10-leading-causes-of-death-among-among-the-hispanic-community/
13. https://minorityhealth.hhs.gov/hispaniclatino-health
14. https://pmc.ncbi.nlm.nih.gov/articles/PMC4840042/
15. https://escholarship.org/uc/item/2nn0386b
16. https://www.cdc.gov/mmwr/pdf/wk/mm64e0505.pdf
17. https://www.cdc.gov/mmwr/volumes/73/wr/mm7331a1.htm
18. https://www.cdc.gov/mmwr/volumes/73/wr/pdfs/mm7331a1H.pdf
19. https://academic.oup.com/jnci/article/116/7/1145/7616986
20. https://d-nb.info/113798175X/34
21. https://acsjournals.onlinelibrary.wiley.com/doi/full/10.3322/caac.21695
.png)
.png)







Comments
Post a Comment