Gut Health 101: How to improve Gut Health (2025 Edition)
Mounting scientific evidence also continues to suggest a large component of nutrition centers on nourishing health-promoting bacteria in your gut (and elsewhere in and on your body). In doing so, you keep harmful microbes in check and shore up your protection against chronic disease.
Disease Begins in Your Gut
But your diet also plays a crucial role. The paper specifically addresses the role of zonulin-mediated gut permeability in the pathogenesis of chronic inflammatory diseases (CIDs). According to the author, Dr. Alessio Fasano,(4) a pediatric gastroenterologist, researcher and director of the Center for Celiac Research and Treatment:(5)
"Apart from genetic makeup and exposure to environmental triggers, inappropriate increase in intestinal permeability (which may be influenced by the composition of the gut microbiota), a 'hyper-belligerent' immune system responsible for the tolerance-immune response balance, and the composition of gut microbiome and its epigenetic influence on the host genomic expression have been identified as three additional elements in causing CIDs.
During the past decade, a growing number of publications have focused on human genetics, the gut microbiome, and proteomics, suggesting that loss of mucosal barrier function, particularly in the gastrointestinal tract, may substantially affect antigen trafficking, ultimately influencing the close bidirectional interaction between gut microbiome and our immune system.
This cross-talk is highly influential in shaping the host gut immune system function and ultimately shifting genetic predisposition to clinical outcome. This observation led to a re-visitation of the possible causes of CIDs epidemics, suggesting a key pathogenic role of gut permeability.
Pre-clinical and clinical studies have shown that the zonulin family, a group of proteins modulating gut permeability, is implicated in a variety of CIDs, including autoimmune, infective, metabolic, and tumoral diseases. These data offer novel therapeutic targets for a variety of CIDs in which the zonulin pathway is implicated in their pathogenesis."
Related: Best Probiotic Supplements
 |
| Mitochondrial Dysfunction Destroys Gut Health |
Bacteria, Not Genes, Rule Your Health Destiny
Aside from the microbes themselves, the condition of your intestinal mucosa also plays a significant role. "Although this enormous mucosal interface (200 m2) is not apparently visible, it plays a pivotal role through its dynamic interactions with a variety of factors coming from our surrounding environment, including microorganisms, nutrients, pollutants and other materials," Fasano explains.
While intracellular tight junctions used to be thought of as static and impermeable, we now know this is not the case. As explained by Fasano, zonulin is a powerful modulator of intestinal permeability. However, while zonulin is a biomarker of gut permeability and plays a pathogenic role in in many chronic inflammatory diseases, not all CIDs are caused by leaky gut.
How Mitochondria Drive Your Gut Health
Many people in the wellness world take a gut-first approach to health, using phrases such as "all disease begins in the gut."If you want to take care of your gut, however, you need to start taking care of your mitochondrial function.
Consider the following:
- The right gut microbiome is nurtured when nutrients were are supposed to absorb are well absorbed and nutrients we are supposed to leave to the microbes are left to the microbes. Absorption of just the right things from our food is an incredibly energy-intensive process, and it is fueled by the mitochondria of our gut cells.
- The microbiome is fine-tuned by the activity of our immune system. The ability of immune cells to differentiate, proliferate, migrate, kill pathogens, and support beneficial microbes are all incredibly energy-intensive processes, and fueled by mitochondria.
- The absorptive cells of our gut are exposed to everything in our diet as they filter what we need to absorb from our food from what could harm us and is best left behind, and they bare the brunt of the toxicity of our environment. As a result, they need to be replaced every 3-4 days. Constantly making new cells is extremely energy-intensive and fueled by mitochondria.
SIBO occurs when bacteria overgrow in the small intestine, often due to malabsorption of nutrients, leaving excess fuel for microbial fermentation. In this case, genetic testing revealed that the individual had a defect in a thiamin-dependent mitochondrial enzyme. By addressing this mitochondrial dysfunction with high-dose thiamin, the underlying issue was resolved, and their SIBO symptoms subsided.
Read More:
- Berberine and rifaximin effects on small intestinal bacterial overgrowth: Study protocol for an investigator-initiated, double-arm, open-label, randomized clinical trial (BRIEF-SIBO study)
- Efficacy of rifaximin in treating with small intestine bacterial overgrowth: a systematic review and meta-analysis
However, high-dose thiamin is not a universal solution. While some individuals experience remarkable benefits, others have reported adverse effects. In some cases, the same regimen has led to new-onset motor disorders or significantly worsened dysautonomia, emphasizing the importance of personalized approaches in supplementation.
Consider some other critical clues from modern research into the science of the gut:
- In classical niacin deficiency, known as pellagra, "diarrhea" is one of the "three Ds" in the mnemonic device that doctors and dieticians have used to remember the symptoms. We now know that the reason diarrhea results is because the microvilli of the small intestine -- tiny fingerlike projections that serve to increase absorptive surface area -- become flattened just like in celiac disease. Why? Because niacin is essential to mitochondrial energy production, and if mitochondria don't produce energy, they cannot support the replication of gut cells. Malabsorption ensues, and the person gets diarrhea.
- In genetic mutations causing clinically diagnosable mitochondrial dysfunction, the mutations will impact gut function to such an extent that, according to the leading textbook in the field (Saudubray, et al), “they are easily misdiagnosed as cow’s milk protein intolerance, celiac disease, chronic ear, nose, and throat infections, late-onset chronic pyloric stenosis [which prevents food from passing from the stomach to the intestine], etc.”
- Constipation is often secondary to Parkinson's, diabetes, and hypothyroidism. Parkinson's is associated with decreased brain perception of the value of investing energy in movement; hypothyroidism is associated with decreased brain perception of the availability of energy to invest in any activity throughout the body; diabetes is associated with decreased capability of burning fuel for energy. These three associations are a huge signal that mitochondrial dysfunction drives gut motility. As noted in the Saudubray textbook, genetic mitochondrial dysfunction can cause constipation, diarrhea, and any form of gut dysmotility.
- A recent study looked at 192 biomarkers as potential predictors of a relapse of Crohn's disease or ulcerative colitis. Only seven biomarkers had predictive power. Three were markers of increasing immune dysfunction, but four were markers of declining mitochondrial function.
When mitochondrial dysfunction is secondary to a problem like Parkinson's, diabetes, or hypothyroidism, this really reflects a vicious cycle rather than the diagnosed disease being a root cause. For example, in lab rats researchers induce Parkinson's by inhibiting complex I of the mitochondrial respiratory chain. The hypothalamus governs thyroid production according to the efficiency of ATP extraction from food -- that is, according to what mitochondria do with your food, not according to how much you eat. Insulin resistance sets in when it is telling cells to burn fuel for energy but their mitochondria cannot keep up with the signal.
Thus, if "all disease begins in the gut" you need to start fueling your gut and its immune system with optimal mitochondrial energy production.
If you are hitting all the basics of good health and your digestion is still feeling sluggish or your gut is failing to serve you the way it should, that's the signal it's time to find out exactly what your mitochondria need.
All disease may start in the gut, but a healthy gut starts with healthy mitochondrial function.
If you know exactly what your mitochondria needs to function, you can move beyond laxatives for constipation, bismuth and fiber for diarrhea, expensive and risky biologics for IBD, antibiotics for SIBO, and endless experimentation with different probiotics looking for the magic link that will finally work for you. Instead, by giving your mitochondria exactly what they need to produce abundant cellular energy, you will nourish your gut and its immune system to maintain the microbiome that is just right for you, optimal nutrient absorption, and well-balanced motility.Proposed Chain of Events Leading to CID (Chronic Inflammatory Disease)
The graphic below, included in Fasano's review but originating from an earlier paper (6) titled "Zonulin, a Regulator of Epithelial and Endothelial Barrier Functions, and Its Involvement in Chronic Inflammatory Diseases," co-written by Fasano and Craig Sturgeon, details the "proposed chain of events leading to chronic inflammatory disease."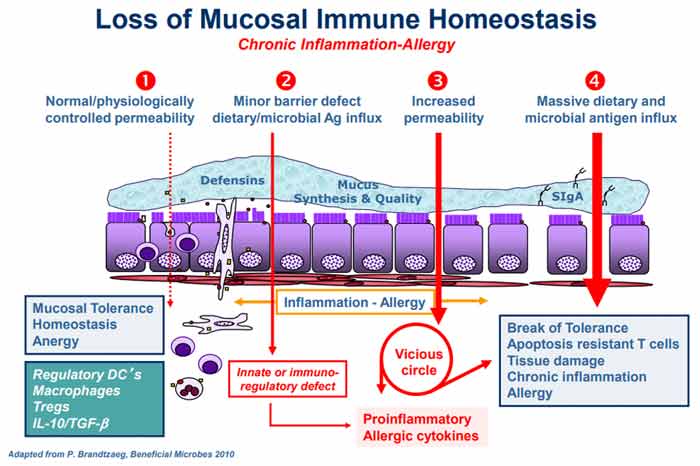
Under normal circumstances, a healthy homeostasis is maintained in your gut lining such that when an antigen is encountered, no excess immune reaction occurs (anergy). Under No. 2 in the graph, gut dysbiosis is setting in (i.e., an imbalance in the number and diversity of your gut microflora), causing excess production of zonulin, which in turn makes the gut lining more permeable.
According to Fasano, the two most powerful triggers of zonulin release are bacteria overgrowth and gluten. Zonulin is produced in response to bad bacteria7 — it helps flush the bacteria out by opening up the tight junctions — so bacteria overgrowth makes sense. But why does it respond to gluten?
Interestingly enough, the zonulin pathway misinterprets gluten as a potential harmful component of a microorganism. That's why gluten triggers zonulin release. While not mentioned by Fasano, the herbicide glyphosate also triggers zonulin, and is 10 times more potent than gluten!8
The subsequent permeability allows microbiota-derived antigen and endotoxin to migrate from the lumen to the lamina propria (the connective tissue that is part of the mucous membrane lining your intestine), thereby triggering inflammation.
As the process continues to worsen (No. 3 in the graph), your adaptive immune response kicks in, triggering the production of proinflammatory cytokines, including interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α). These cytokines further worsen the permeability, thus creating a vicious cycle. Eventually (No. 4), mucosal tolerance is completely broken, resulting in the onset of a chronic inflammatory disease.
Chronic Inflammatory Diseases Linked to Leaky Gut
The specific chronic inflammatory disease that ultimately emerges at the end of all this depends in part on your genetic makeup, in part on the types of exposures you've had, and in part on the composition of your gut microbiome. As explained by Fasano:(9)"Besides genetic predisposition and exposure to environmental triggers, the pathogenesis of a variety of CIDs seems to involve mutually influenced changes in gut permeability/Ag trafficking, immune activation, and changes in composition/function of the gut microbiome.
Zonulin is a modulator of both epithelial and endothelial barrier functions … Gut dysbiosis may cause the release of zonulin leading to the passage of luminal contents across the epithelial barrier causing the release of pro-inflammatory cytokines that themselves cause increased permeability establishing a vicious loop leading to massive influx of dietary and microbial Ags triggering the activation of T cells.
Depending on the host genetic makeup, activated T cells may remain within the GI tract, causing CID of the gut … or migrate to several different organs to cause systemic CID."
- Autoimmune disorders such as Celiac disease, Type 1 diabetes, inflammatory bowel disease, multiple sclerosis and ankylosing spondylitis
- Metabolic disorders such as obesity, insulin resistance, nonalcoholic fatty liver disease, gestational diabetes, hyperlipidemia and Type 2 diabetes
- Intestinal diseases such as irritable bowel syndrome, non-celiac gluten sensitivity and environmental enteric dysfunction (a chronic disease affecting the proximal intestine)
- Neuroinflammatory diseases such as autism spectrum disorder, schizophrenia, major depressive disorder and chronic fatigue/myalgic encephalomyelitis
- Brain and liver cancers
Gut Microbes Influence Genes and Can Influence Cancer Risk
Not only have gut bacteria been shown to influence gene expression,(10,11) turning some genes on and others off, research(12) published in 2018 found gut microbes actually control antitumor immune responses in your liver, and that antibiotics can alter the composition of immune cells in your liver, triggering tumor growth.
Certain gut bacteria also promote inflammation, which is an underlying factor in virtually all cancers, whereas other bacteria quell it.(13) The presence of certain gut bacteria has even been shown to boost the patient's response to anticancer drugs.(14)
One way in which gut bacteria improve the effectiveness of cancer treatment is by activating your immune system and allowing it to function more efficiently. Researchers have actually found that when these specific microbes are absent, certain anticancer drugs may not work at all.
Gut Bacteria Are Part of Your Antiviral Defense
"For the first time, Harvard Medical School researchers have … identified the specific population of gut microbes that modulates both localized and systemic immune response to ward off viral invaders. The work … pinpoints a group of gut microbes, and a specific species within it, that causes immune cells to release virus-repelling chemicals known as type 1 interferons.
The researchers further identified the precise molecule — shared by many gut bacteria within that group — that unlocks the immune-protective cascade. That molecule, the researchers noted, is not difficult to isolate and could become the basis for drugs that boost antiviral immunity in humans."
While the findings still need to be replicated and confirmed, they point to the possibility that you might be able to enhance your antiviral immunity by reseeding your gut with Bacteroides fragilis and other bacteria in the Bacteroides family.17
These bacteria initiate a signaling cascade that induces the release of interferon-beta that protect against viral invasion by stimulating immune cells to attack the virus and causing virus-infected cells to self-destruct.
The Role of Vitamin D
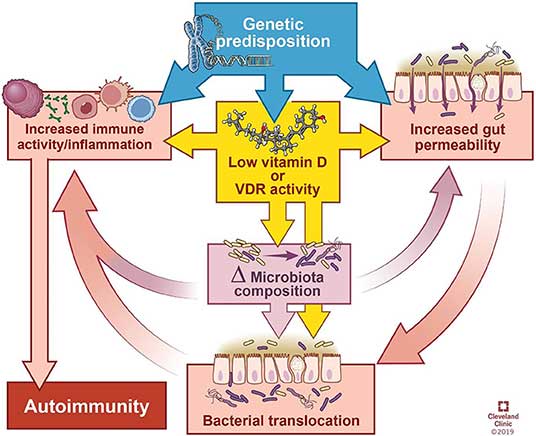
"Autoimmune diseases tend to share a predisposition for vitamin D deficiency, which alters the microbiome and integrity of the gut epithelial barrier.
In this review, we summarize the influence of intestinal bacteria on the immune system, explore the microbial patterns that have emerged from studies on autoimmune diseases, and discuss how vitamin D deficiency may contribute to autoimmunity via its effects on the intestinal barrier function, microbiome composition, and/or direct effects on immune responses."
As noted in this review, vitamin D has several direct and indirect regulatory effects on your immune system, including promoting regulatory T cells (Tregs), inhibiting differentiation of Th1 and Th17 cells, impairing development and function of B cells, reducing monocyte activation and stimulating antimicrobial peptides from immune cells.
That said, the relationship between vitamin D and autoimmunity is complicated. Aside from immunosuppression, vitamin D also appears to improve autoimmune disorders by the way it affects your microbiota composition and gut barrier.
The review cites research showing that your vitamin D status alters the composition of your gut microbiome. Generally speaking, vitamin D deficiency tends to increase Bacteriodetes and Proteobacteria while higher vitamin D intake tends to increase prevalence of Prevotella and reduce certain types of Proteobacteria and Firmicutes.
While research is still slim when it comes to vitamin D's impact on gut bacteria, especially in patients with autoimmune disease, vitamin D deficiency and autoimmune diseases are known comorbidities and vitamin D supplementation is often recommended for these patients.
Vitamin D Required for Tight Junction Maintenance
“The intestinal epithelium is in constant interaction with the external environment. Adequate barrier integrity and antimicrobial function at epithelial surfaces are critical in maintaining homeostasis and preventing invasion or overcolonization of particular microbial species.
A healthy intestinal epithelium and intact mucus layer are critical to protect against invasion by pathogenic organisms, and vitamin D helps to maintain this barrier function … Multiple studies found that vitamin D3/VDR signaling modulates tight junction protein quantity and distribution …
As a ‘leaky’ protein that allows movement of ions into the intestinal lumen, claudin-2 expression in the setting of functional vitamin D deficiency may contribute to colitis pathology …
Vitamin D upregulates antimicrobial peptide mRNA and protein expression including cathelicidin, defensins, and lysozyme … Antimicrobial peptides, primarily secreted by Paneth cells in the gut, are important mediators of microbiome composition … Defensins are secreted by epithelial cells, Paneth cells, and immune cells, and are important components of the innate immune response in the gut.”
How Vitamin D Deficiency May Contribute to Autoimmune Disease
- Vitamin D deficiency or supplementation changes the microbiome, and manipulation of bacterial abundance or composition impacts disease manifestation.
- Lack of vitamin D signaling due to dietary deficiency can impair physical and functional barrier integrity of the gut, thereby allowing bacterial interactions to either stimulate or inhibit immune responses.
- Your innate immunologic defenses may be compromised if you are deficient in vitamin D.
How to Optimize Your Gut Microbiome
Regularly eating traditionally fermented and cultured foods is the easiest, most effective and least expensive way to make a significant impact on your gut microbiome. Healthy choices include lassi (an Indian yogurt drink), cultured grass fed organic milk products such as kefir and yogurt, natto (fermented soy) and fermented vegetables of all kinds.
Although I'm not a major proponent of taking many supplements (as I believe the majority of your nutrients need to come from food), probiotics are an exception if you don't eat fermented foods on a regular basis. Spore-based probiotics, or sporebiotics, can be particularly helpful when you're taking antibiotics. They're also an excellent complement to regular probiotics.
Sporebiotics, which consist of the cell wall of bacillus spores, will help boost your immune tolerance, and because they do not contain any live bacillus strains, only its spores — the protective shell around the DNA and the working mechanism of that DNA — they are unaffected by antibiotics.
Antibiotics, as you may know, indiscriminately kill your gut bacteria, both good and bad. This is why secondary infections and lowered immune function are common side effects of taking antibiotics. Chronic low-dose exposure to antibiotics through your food also takes a toll on your gut microbiome, which can result in chronic ill health and increased risk of drug resistance. Last but not least, you also need to avoid things that disrupt or kill your microbiome, and this includes:
- Antibiotics, unless absolutely necessary
- Conventionally-raised meats and other animal products, as these animals are routinely fed low-dose antibiotics, plus genetically engineered and/or glyphosate-treated grains
- Processed foods (as the excessive sugars feed pathogenic bacteria)
- Chlorinated and/or fluoridated water
- Antibacterial soap and products containing triclosan
Coconut Water and Gut Health
The Good News: Gut Health Is Highly Adaptable
One of the most encouraging aspects of gut health is its ability to regenerate and adapt quickly! The gut lining is replaced every three days16 and the gut microbiome can change within a single day based on dietary choices.When increasing carbohydrate intake, it's important to start slowly and focus on whole food sources. Pay attention to your individual response to avoid digestive discomfort and try to maintain consistent as you reintroduce. Best sources of gut-friendly carbohydrates:
- Rice
- Sourdough bread, or quality made bread using well-sourced grains
- Root vegetables like potatoes and sweet potatoes (if you can find Japanese sweet potatoes or purple sweet potatoes, they taste so much better than the orange variety!)
- Fresh, ripe fruits
- Masa harina, or traditionally made tortillas
How to Improve Gut Health Naturally
1. Fiber-rich foods2. Probiotics and Fermented Foods
3. Prebiotics
4. Hydration
6. Regular Exercise
7. Adequate Sleep
The Gut-Brain Axis: Connecting the dots between Gut Health and Brain Health
The gut-brain axis is another crucial factor in the diabetes-dementia connection. This bidirectional communication system between your gut and brain plays a significant role in both metabolic and cognitive health. Your gut microbiota, the trillions of microorganisms living in your intestines, are key mediators in this relationship.In diabetes, there's often a state of gut dysbiosis — an imbalance in the microbial community. This dysbiosis leads to increased intestinal permeability, often called "leaky gut," allowing harmful substances to enter your bloodstream and trigger systemic inflammation.
Similarly, alterations in gut microbiota composition have been observed in Alzheimer's disease patients. These changes affect the production of neurotransmitters, immune responses and even the integrity of your blood-brain barrier. Intriguingly, some gut bacteria produce compounds that mimic amyloid proteins, potentially exacerbating Alzheimer's pathology (source).
Meanwhile, fostering beneficial oxygen-intolerant bacteria in your gut, including important species like Akkermansia, strengthens your intestinal defenses and promotes overall wellness. These beneficial bacteria ferment dietary fibers to produce short-chain fatty acids (SCFAs), particularly butyrate.
Notably, butyrate-producing bacteria like Eubacterium and Eisenbergiella were associated with lower Alzheimer's risk. Butyrate nourishes your colonic epithelial cells, reinforcing the intestinal barrier. SCFAs also stimulate mucin production, creating a protective shield against harmful bacteria.
Diets rich in polyunsaturated fats (PUFAs), including linoleic acid found in seed oils, destroy your gut health, leading to a cascade of harmful effects, from Type 2 diabetes to Alzheimer’s.
Can Broccoli Help Your Gut?
Good Starch vs Bad Starch
One of the primary functions of the gut is to maintain an anaerobic environment (an environment without oxygen). The problem is, you need energy to keep oxygen out of there, and if that energy is not available, oxygen is going to seep in.Most beneficial bacteria are gram-negative, and they're called obligate anaerobes. They do not have LPS in their cell wall and hence will not produce endotoxin when they die off. However, when you don’t create enough cellular energy you are unable to create a low oxygen environment in your large intestine.
This kills the beneficial bacteria as oxygen seeps in and they are unable to survive. When they leave, they create a hole that allows endotoxin-producing bacteria — facultative anaerobes — to take over through competitive inhibition. Facultative anaerobes can tolerate oxygen and survive.
The primary obligate anaerobic bacterium in your gut is a species called Akkermansia, which makes mucin, the protective layer in your gut. When your Akkermansia die off due to lack of cellular energy to maintain the proper oxygen gradient in the large intestine, then your mucin barrier starts to break down and you end up with leaky gut.
Now, the reason starch CAN be problematic is because, if you are metabolically inflexible (and most are), then you’re not making enough mitochondrial energy to maintain a healthy gut. So, the idea that starch is problematic is likely true for most people, because most people have a disrupted microbiome. Starch is indiscriminate and will feed any bacteria. So, since most people have a preponderance of pathogenic gut bacteria, starch causes problems.
The flip side of this is that if you have a healthy microbiome, starch can be quite beneficial. So, the primary goal is to get your cellular energy up and improve your microbiome first. Then you can eat starch.
Carbs and Gut Health: A Color-Coded System to Guide Your Gut Health Journey
The method that Dr Joseph Mercola discuss in his book ranks carbohydrates based on their impact on your biology, specifically in relation to your gut health. This approach recognizes that the traditional complex vs. simple carb dichotomy likely does not tell the whole story when it comes to individual health outcomes.Instead, it suggests that the relationship between your gut health and carbohydrate metabolism could be key to unlocking improved overall wellness. It's not about following a one-size-fits-all diet, but rather about understanding how your unique gut biology interacts with different types of carbohydrates.
Surprisingly, for many people, this approach favors simple carbs over complex ones. This is because they usually have less-than-optimal gut health. If you have a compromised gut system and you consume complex carbs, the fiber and prebiotics in these carbs can feed oxygen-tolerant gut bacteria and worsen your symptoms.
The following chart breaks down several types of carbohydrate sources and how they fit into this plan. We can categorize them into three groups: green, yellow and red.

In the green category are the most easily digestible simple carbs that provide quick energy without overtaxing your compromised digestive system. You will focus on these carbs initially, because simple carbs provide a quick energy boost for your cells and mitochondria. It's like giving your body's energy factories an immediate fuel injection while allowing your gut to rest and heal at the same time.
Next is the yellow category, which includes carbs that offer more nutrients and fiber compared to the green category, yet are still relatively easy on the digestive system. Finally, the red category, the most complex carbs, offers many health benefits but can be challenging for a compromised gut to handle.
So how can you begin implementing this approach? If you have severely compromised gut health, start with pure sugar water. This is a temporary measure to jumpstart the healing process. Mix one-half pound, up to a full pound, of pure dextrose (glucose) into a half gallon of water and sip it slowly all day. Don't drink more than an ounce at a time to avoid spiking your insulin.
Once your gut health has improved, you can switch your primary carb source to whole foods. More than likely, you'll also need to eat more frequently than you're used to during this transition to avoid hypoglycemia. Eating every three to four hours, with snacks throughout the day, is crucial when relying on simple carbs for energy.
As your mitochondrial energy production continues to improve and your gut starts to heal, you will begin the transition back to complex carbs. This is a slow and steady process — don't rush it.
Once you're able to include more complex carbohydrates in your diet, you'll start to notice significant benefits. You'll be able to extend the time between meals to between four and six hours, and many people find they can comfortably switch to a three-meals-a-day approach. This is because complex carbs digest more slowly, providing a steady stream of energy.
Key Takeaways
The best path forward is to remove obstacles that harm your mitochondria — restoring your cellular energy production — then supply the beneficial carbohydrates your gut thrives on. If you’re somebody with chronic digestive problems, addressing the root cause is far more effective than any single diet hack or short-term plan. Below are five steps toward that end:- Remove seed oils and other mitochondrial poisons — If you eat out frequently or consume processed foods, you’re getting a load of linoleic acid from seed oils like sunflower, safflower, soybean and canola. Learn to decipher food labels and identify hidden sources of omega-6s. Look for ingredients like canola oil, safflower oil, corn oil or soybean oil and any of their derivatives. These oils disrupt how your cells make energy, which ultimately wrecks your gut environment. Switch to butter, ghee or tallow instead.
- Avoid endocrine disruptors and EMFs — Plastics and common household items release chemicals that interfere with your body’s hormones. These are often called endocrine-disrupting chemicals. Store your foods in glass or stainless steel containers instead of plastic when possible. Keep in mind that electromagnetic fields (EMFs) also stress your energy production. Consider small tweaks like turning off Wi-Fi at night or using wired connections, especially if you struggle with chronic inflammation or fatigue.
- Start with easily digestible carbohydrates — Individuals with severely compromised gut health often tolerate simpler carb sources better than complex options. If your gut health is compromised, start with dextrose water, sipped slowly throughout the day. As you feel better, gradually add in whole fruit or juice with pulp. White rice is next on the list before heavier starches or fibrous vegetables. By taking small steps, you reduce the risk of bloating, discomfort and unwanted endotoxin buildup.
- Introduce Akkermansia supplements wisely — Akkermansia muciniphila is a key bacterium that strengthens your gut barrier, but most people have very low levels. After you eliminate seed oils for at least half a year, consider adding a timed-release Akkermansia supplement. This allows more of the bacteria to survive and reach your colon. Don’t rush into supplementation if you’re still consuming processed foods that sabotage your microbiome. Give your gut environment the best possible chance to welcome those beneficial microbes by eliminating seed oils first, then adding in Akkermansia via timed-release capsules or microencapsulation technology.
- Slowly reintroduce fiber and starches — Once your gut is healthy, add fibrous vegetables and grains in small amounts. If you are someone who lifts weights or runs frequently, your carb requirement goes up, so it makes sense to expand options like fruits, cooked vegetables and starchier foods. Keep an eye on your body’s response. Too much fiber too soon ramps up endotoxin release and triggers digestive issues. Gradual changes give your gut time to adapt without unpleasant side effects.
- 1 Hopkinsmedicine.org, The Gut: Where Bacteria and Immune System Meet
- 2 PLOS ONE February 5, 2010; 5(2): e9085
- 3, 5, 9 F100Research 2020, 9(F1000 Faculty Rev):69
- 4 IFM.org Alessio Fasano
- 6 Tissue Barriers 4:4, e1251384
- 7, 8 Biomed Network, Zonulin, Gluten, Glyphosate and Tight Junctions
- 10 Mol Cell. 2016 Dec 1;64(5):982-992.
- 11 ScienceDaily November 23, 2016
- 12 NIH.gov, May 24, 2018
- 13, 14 Nature May 23, 2018
- 15 Cell November 25, 2020; 183(5): 1312-1324
- 16, 18 Harvard Medical School November 18, 2020
- 17 Bacteroides Fragilis
- 19, 20 Frontiers in Immunology January 21, 2020 DOI: 10.1189/fimmu.2019.03141


.png)
.png)






Comments
Post a Comment