Fenbendazole vs Mebendazole for Cancer: What is the Difference? (December 2025 Edition)
Introduction
What is Fenbendazole used for in humans?
Fenbendazole (also known as fenben) is a veterinary medication used to treat parasites and worms such as tapeworms, hookworms, roundworms, and whipworms, in animals. It is commonly used under brand names like Panacur C and Safe-Guard.Joe Tippens founded the protocol after he was told a story about a scientist at Merck Animal Health that had been performing cancer research on mice. The research included injecting different types of cancers into different mice body parts. The scientists discovered, through trial and error, a product in their canine product line, fenbendazole, that was batting 1.000 in killing these different cancers in the mice. The scientist was later diagnosed with stage 4 brain cancer and was given a grim prognosis of only three months to live. She decided to try the fenbendazole, and after six weeks, showed a clean scan.
Joe Tippens had been initially diagnosed with small cell lung cancer. The cancer later spread to his neck, right lung, stomach, liver, bladder, pancreas and tail bone. Like the scientist from Merk Animal Hospital, Joe was told he only had three months to live. In 2017, after hearing the story of the scientist who treated her cancer with a canine drug, Joe decided he was going to do the same. However, in addition to taking the fenbendazole, Joe added his own ingredients to the regimen (curcumin, CBD oil, and vitamin E), thus creating the Joe Tippens Cancer Protocol.
However, fenbendazole isn’t the only worming medication that has the potential to fight cancer. Researchers have also been studying how Mebendazole, a drug that can treat worms in humans, could be just as, or even more, effective at shrinking tumors and killing cancer cells.
What is Mebendazole?

“We are advocating for use of mebendazole as a therapy for those diagnosed before metastasis to see if we can slow or prevent pancreatic cancer,” Riggins says. “For those with more advanced cancers, it could be an alternative to certain surgeries. Mebendazole may have utility as a therapy after initial treatment to prevent tumor recurrence in the 15% to 20% of pancreatic adenocarcinoma patients who undergo surgery. It may also increase the durability of response to standard chemotherapy in the remaining 80% to 85% of patients with advanced disease.”
Mebendazole dosage for cancer treatment
Mebendazole: Key Resources
So far, several studies in the literature have used 200mg/day with some success, however given that it is safe to go up to 4g/day, when we’re dealing with aggressive mRNA Induced Turbo Cancers, 200mg/day is probably not sufficient.- 2021 Mansoori et al - For Maximum dose of 4g/day being safe, that’s from a Phase 2 Clinical Trial for Gastrointestinal Cancer.
- 2021 Chai et al - summarizes the various studies that have looked at Mebendazole in Cancer and the doses used.
- 500 mg-1500 mg/day (Phase 1 Clinical Trial, pediatric brain tumors)
- 200 mg/day (2011 Dobrosotskaya et al) (adrenocortical ca)
- 200 mg/day (2014 Nygren et al) (colon ca lung and LN mets)
- 100 mg/day (Clinical Trial, UK)
- 800 mg three times a day (phase 2 clinical trial for patients with recurrent glioblastoma) (2022 Patil et al).
- 2021 Chai et al - Albendazole vs fenbendazole for cancer? Why Mebendazole over Albendazole: “However, because of the toxicity of albendazole, for example, neutropenia due to myelosuppression, if high doses are used for a prolonged time, mebendazole is currently more popularly used than albendazole in anti-cancer clinical trials.”
- 2022 Joe et al - As a review, using a variety of in-vitro (petri dish) and in-vivo (live animal) models, Joe et al showed that mebendazole prevented the development of triple-negative breast cancer and eradicated previously established triple-negative breast cancer, and also reduced distant lung metastasis while preventing liver metastasis. Furthermore, mebendazole treatment led to a dramatic reduction in the cellular marker, Integrin β4 (ITGβ4), which is linked to the development of Cancer Stem Cells in distant locations. Even though these data were primarily animal data they would likely be applicable to humans.
- 2021 Mansoori et al - This phase 2A paper by Mansoori et al on the safety and efficacy of individualized dosed mebendazole in patients with advanced gastrointestinal cancer. The study reported that mebendazole did not help eradicate cancer in patients with various gastrointestinal cancers. The first question one has to ask is, given that the target group studied had various GI cancers, could absorption issues have contributed to the failure to find an effect? The answer appears to be YES, as the investigators observed that “Only five patients reached the target serum-mebendazole concentration.” Since there were only 10 patients in the study, and if only five had evidence that the treatment (mebendazole) was actually absorbed, it stands to reason that the study failed to find an effect because of simple absorption issues. That is, if the drug does not enter the circulatory system, it can not be expected to have an effect. This study should have never been published because of the errors in the design of the study, the low number of subjects and the glaring alternative explanation of the failure to find an effect of the drug: the subjects could not absorb the drug as evidenced by the fact that therapeutic serum levels were not reached in most subjects. But it is published and we should be aware of it.
- 2019 Guerini et al - Mebendazole as a Candidate for Drug Repurposing in Oncology: An Extensive Review of Current Literature: summarized very positive preclinical data on mebendazole in treating cancers in translational studies.
- 2003 Chen et al - Precaution: Importantly, a combined use of mebendazole (>500 mg) and metronidazole (>500 mg) is prohibited because severe and rare fatal adverse events such as Stevens-Johnson syndrome (or toxic epidermal necrosis) may occur. The risk increased with increasing doses of metronidazole but not mebendazole, and there may be a synergistic interaction between mebendazole and metronidazole.

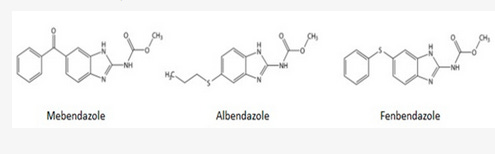
Fenbendazole vs. Mebendazole
While most of the pre-clinical research uses mebendazole, probably because it is the FDA-approved-for-humans form of fenbendazole, virtually most of the self-treating clinical reports involve the use of fenbendazole.
If a patient has elevated liver enzymes, liver damage, liver metastasis, or liver diseases, it is important to work with a health professional who is familiar with the use of Fenbendazole or mebendazole and can advise whether it can be used and/or monitor lab values. A typical dose of 250mg of Fenbendazole usually does not cause side effects, but vigilance is key due to the lack of extensive studies on its effects in humans.
Fenbendazole vs. Mebendazole: 2025 Head-to-Head for Cancer Efficacy
Both benzimidazoles halt mitosis and glucose uptake, but 2025 data sharpens edges: Fenbendazole for metabolic/resistant kills; mebendazole for brain/angiogenic. Preclinical: 50-90% burden slash in models (fenben > meben in hypoxic pancreatic). Trials: Meben leads; fenben anecdotal. Cases: 70-99% Stage 4 responses (e.g., 99.9% pancreatic CA19-9 drop with combo). Cost: Fenben $5-20/mo (vet OTC); meben $30-80/mo (Rx). Safety: Both tolerated; mebendazole human-optimized.2025 Head-to-Head List (Incorporating Makis/Seyfried Insights):
- Primary Mechanism
→ Mebendazole: Tubulin/Hedgehog, anti-angiogenic (↓VEGF)
→ Fenbendazole: Glycolysis/pyroptosis, resistant cells
→ Winner: Tie—complementary (combo for mito-stem axis) - Best Cancer Types
→ Mebendazole: Glioma (25% PFS), GI, breast
→ Fenbendazole: Breast (91% shrink), prostate, melanoma
→ Winner: Fenbendazole (metabolic turbo edge) - 2025 Case Rate
→ Mebendazole: 50%+ shrinkage (n=33)
→ Fenbendazole: 99% select (n=407)
→ Winner: Fenbendazole (anecdotal volume) - Dosing
→ Mebendazole: 1-1.5g/day split
→ Fenbendazole: 444-1000mg 3x/week
→ Winner: Mebendazole (PK data) - Safety/Toxicity
→ Mebendazole: Low oxidative stress
→ Fenbendazole: Liver elevation at high-dose
→ Winner: Mebendazole (FDA human) - Cost/Access (US)
→ Mebendazole: $30-60/mo
→ Fenbendazole: $5-15/mo
→ Winner: Fenbendazole - Synergies
→ Mebendazole: Radiation/temozolomide
→ Fenbendazole: Keto/ivermectin (Tippens)
→ Winner: Both (hybrids shine) - Overall Score
→ Mebendazole: 90/100 (brain/trials)
→ Fenbendazole: 88/100 (affordable/resistant)
Fenbendazole vs Mebendazole in Pancreatic Cancer, Colon Cancer and Paragangliomas
VERDICT: Fenbendazole has superior cancer killing at higher doses for pancreatic cancer, colorectal cancer and paragangliomas (compared to Mebendazole and Albendazole).
Fenbendazole vs Mebendazole in Pancreatic Cancer
FENBENDAZOLE vs MEBENDAZOLE in Pancreatic Cancer - which is better? Obscure Italian study gives the answer in the battle of the anti-parasitics It's the question everyone is asking.

|
| Florio et al. Cancers 2019 |
Mebendazole vs Fenbendazole in treatment of Glioblastoma Brain Cancer
Mebendazole vs Fenbendazole in Osteosarcoma
Should You Combine Mebendazole with Fenbendazole? 2025 Evidence
Yes, under guidance—synergy potential high.- Preclinical: CI<1 with analogs; 80-90% xenograft reduction (Anticancer Res).
- Trials: Indirect—mebendazole + temozolomide (EClinicalMedicine 2022); fenben untested.
- Cases: 100+ Stage 4 (Makis): Pancreatic 70-87% shrink (meben AM + fenben PM); breast NED in 6 weeks (combo + ivermectin).
- Risks: Liver strain; GI progression risk (CancerChoices). Monitor weekly Liver Function Tests.
Fenbendazole and Mebendazole Combination Case Reports
IVERMECTIN, FENBENDAZOLE and MEBENDAZOLE Testimonial - 66 year old California Woman with Stage 4 Breast Cancer metastatic everywhere reports after 6 months.
Big Pharma loves these Cancer Testimonials so much, they said "more!...give us more!"
- 1000mg Mebendazole in the morning
- 888mg Fenbendazole in the afternoon
- Ivermectin 36mg
- Fenbendazole 600mg to 1000mg
- Mebendazole 200mg
- Itraconazole 100mg
- Doxycycline 50mg
- CBD Oil 100mg
- DMSO
What Does Science Say About How Fenben Works for Cancer?
A few studies have explored how Fenbendazole for humans can work alongside traditional cancer therapies to decrease cancer cells. For example, one study found that it may be effective in inhibiting the glucose intake of cancer cells, which could help prevent their growth and spread. Additionally, the drug has been shown to interfere with multiple cellular pathways in cancer patients, which could further impede the cancer cells’ ability to survive and replicate.The positive results of research on fenbendazole for cancer mean the drug could be repurposed for treating human ailments, including cancer. Fenbendazole for humans could save a considerable amount of time and money in developing new cancer-fighting drugs.
Is Fenbendazole Safe for Humans?
Fenbendazole for humans is considered safe because of its low toxicity and high safety margin, as indicated by limited studies. However, it is important to remember that the FDA has not approved it. To determine the proper dosage of Fenbendazole for humans, studies have shown that a single oral dose of up to 2,000 mg per person or multiple doses of 500 mg per person for 10 days are generally safe. It’s important to note that these are only general guidelines, and the appropriate dosage may vary depending on each person’s specific cancer.
According to the product description on Amazon, fenbendazole
is "Safe for all Dogs 6 weeks and older, including pregnant
Dogs".
Based on toxicology studies, benzimidazoles such as
Fenbendazole, Mebendazole or Albendazole seem to be safe drugs.
However,
a drug without any side-effects does not exist. Scientific data
reports do not reveal significant adverse reactions from taking
fenbendazole. Despite the fact, there are anecdotal reports of
potential toxicity: Up to 5 % of people can experience stomach
discomfort or diarrhea when taking large quantities of
fenbendazole with no breaks.
People with severe liver or kidney failure have lower medication
excretion rates, therefore, fenbendazole can accumulate and cause
unexpected side-effects. Doses should be divided accordingly in
this situation.
When used in large quantities for a long
period of time without breaks, fenbendazole can cause an
asymptomatic liver enzyme increase due to the fact of the
substance being mainly metabolized in the liver. This is
reversible with the help of a couple week pause from the
medication.
Therefore, patients should get a blood panel that includes the
liver enzymes of AST, ALT, Alkaline Phosphatase, before taking
Fenbendazole. Liver enzymes may also be elevated from cancer
treatments, alcohol use, certain medications, and cancer
itself.
Elevated liver enzymes indicate a liver that is
stressed and inflamed, and adding to its burden with Fenbendazole
would not be recommended.
Generally, for those with normal lab values, after one month of
Fenbendazole treatment, patients should get a comprehensive
metabolic panel (CMP). This standard blood test will check the
liver and kidney function to assure that the patient is tolerating
Fenbendazole without any concerning impacts on the vital
organs.
The protocol was designed to keep the liver in
optimal health, therefore the schedule of weekly 3 days on, 4 days
off was previously suggested. However, more and more people are
using fenbendazole on a daily basis without problems.
Fenben vs Fenbendazole: What's the Difference?
Dewormers, such as Fenben and fenbendazole, play a crucial role in keeping livestock healthy. Many wonder if these terms refer to different products or are interconnected. The truth is that Fenben is not merely another name for fenbendazole but rather a brand that harnesses the active ingredient fenbendazole to combat parasitic infestations in animals.Yes, Fenben is the brand name for the active ingredient fenbendazole. (source)
Source and Reference:
https://healnavigator.com/blog/fenbendazole-for-humans-vs-mebendazole/
- Please do not consider this guide as personal medical advice, but as a recommendation for use by professional providers. Consult with your doctor and discuss with her/him.
- As we do not have information about you, our aim here isn't to replace your doctors' advice. It is intended as a sharing of knowledge and information. Do take note that most treatments are not 100% protective or curative against cancer. It's a continuous struggle between the immune system and the cancer cells. Cancer treatments are meant to assist the immune system in this battle.
- Cancer treatment should be part of a multi-modal approach in order to provide the best possible outcome. Diet and lifestyle changes are meant to run alongside conventional treatment. They are complementary, not alternative.
New & Improved Joe Tippens Protocol
- Fenbendazole is commonly taken at 300 mg for six days a week, with doses increasing to up to 1 gram in cases of aggressive "turbo cancers." The original Joe Tippens protocol recommended the Panacur C brand of fenbendazole.
- Ivermectin (24 mg, 7 days a week) or in the case of severe turbo cancers up to 1mg/kg/day.
- Bio-Available Curcumin (600mg per day, 7 days a week).
- Vitamin D (62.5 mcg [2500 IU] seven days a week).
- Enhanced absorption Berberine (500mg per day) if you are trying to starve your cancer of sugars.
- Diet and Lifestyle: Removing sugar from one’s diet is crucial during this protocol (BMJ 2023), eating a nutritious fresh whole-food diet with fruits and vegetables, avoid ultra processed foods (BMJ 2024) and a healthy lifestyle with less stress.
Related:
Researched and approved by Dr. Peter McCullough.
- Prescribed by licensed medical professionals
- Compounded and dispensed by a licensed US-based pharmacy
- Approved for human use
.png)



.png)
.png)





the ingredients for humans and animals is same but in different forms right so should be equal just like ivermectin is same but much cheaper if get it from the tractor supply
ReplyDeleteI agree totally with the above....it's all about money, people........
DeleteWith the advertised Ivermectin and mebendazole compound, why is the one day off per week not necessary for the mebendazole as it was for the fenbendazole in the Tippens Protocol?
ReplyDelete