Stage 4 Pancreatic Cancer: Best Combination Treatment (2025)
On this Page:
- What Is Stage IV Pancreatic Cancer?
- What Are Treatments for Stage IV Pancreatic Cancer?
- Key effective chemotherapy combinations
- NALIRIFOX
- FOLFIRINOX
- Targeted Therapy and Immunotherapy
- Ivermectin and Fenbendazole: Repurposed Drugs for Stage 4 Pancreatic Cancer
- Questions to Ask Your Doctor when Diagnosed with Stage IV Pancreatic Cancer
What Is Stage IV Pancreatic Cancer?
Pancreatic cancer is among the most aggressive and difficult cancers to treat,
particularly in its later stages. The prognosis remains poor, making the
development of improved therapies critical. Approximately 80% of
pancreatic cancers are pancreatic ductal adenocarcinomas (PDAC), a subtype
often diagnosed late, which complicates effective treatment. Notably, over
90% of PDAC cases harbor activating mutations in the KRAS gene, a key
driver of tumor development and progression.
Doctors use staging to describe the cancer’s size and location. Stage IV means the
cancer metastasized, or spread, to another part of the body. The tumors
may be any size.
Cancer at this stage may also be called metastatic or advanced cancer.
Most pancreatic cancer patients are diagnosed at stage IV. Patients diagnosed at an earlier stage can also develop stage IV cancer if it spreads.
Cancer at this stage may also be called metastatic or advanced cancer.
Most pancreatic cancer patients are diagnosed at stage IV. Patients diagnosed at an earlier stage can also develop stage IV cancer if it spreads.
Why Is Staging Needed?
The cancer’s stage helps your doctors figure out your treatment choices.Where Does Stage IV Pancreatic Cancer Spread?
Pancreatic cancer often spreads to the:- Liver
- Abdominal wall
- Lungs
- Bones
- Faraway lymph nodes
What Are Treatments for Stage IV Pancreatic Cancer?
The cancer cells are in many places throughout the body and the cancer
cannot be removed by surgery (unresectable) at this stage.
Pancreatic cancer has a high mortality rate and frequently shows resistance
to conventional therapies. Standard treatment options include surgical
resection, chemotherapy, and radiation therapy; however, survival rates
remain low. Recently, alternative strategies, such as repurposing existing
drugs originally developed for non-cancer conditions, have gained
attention.
The best conventional combination treatments for stage 4 pancreatic cancer
currently involve multi-drug chemotherapy regimens that have demonstrated
improved survival compared to older standards.
Key effective chemotherapy combinations
- NALIRIFOX: A four-drug regimen consisting of liposomal irinotecan, 5-fluorouracil/leucovorin, and oxaliplatin. This combination demonstrated longer overall survival (11.1 months) compared to the two-drug regimen nab-paclitaxel plus gemcitabine (9.2 months) in a large Phase 3 clinical trial for metastatic pancreatic ductal adenocarcinoma
- FOLFIRINOX: A combination of 5-fluorouracil (5-FU), leucovorin, irinotecan, and oxaliplatin. This regimen is a standard of care for metastatic pancreatic cancer and has been shown to extend survival compared to gemcitabine alone. Gemcitabine plus a taxane (e.g., nab-paclitaxel): This combination also improves overall survival compared to gemcitabine alone, though fluoropyrimidine-based combinations like FOLFIRINOX or NALIRIFOX may offer further benefits.
Targeted Therapies and Immunotherapy
- Targeted therapies and immunotherapy may be options for patients with specific genetic mutations (e.g., BRCA1/2, PALB2) or biomarkers. Trials combining PARP inhibitors with immunotherapy agents like nivolumab or pembrolizumab plus olaparib have shown promising results in select patients.
- Palliative care and symptom management are important components of treatment to improve quality of life in stage 4 disease. Clinical trials are strongly recommended to access emerging therapies and combinations beyond standard chemotherapy e.g. potential role of Fenbendazole and Ivermectin (see below) in Stage 4 Pancreatic Cancer.
NALIRIFOX vs. FOLFIRINOX for Stage 4 Pancreatic Cancer
Efficacy
Recent studies and real-world analyses have compared the effectiveness of NALIRIFOX and FOLFIRINOX as first-line treatments for metastatic (stage 4) pancreatic cancer:- NALIRIFOX has shown a numerically higher median overall survival (OS) of 11.1 months (95% CI, 10.0–12.1) in some studies.
- FOLFIRINOX has a median OS ranging from 9.1 to 11.7 months (95% CI, 7.8–10.9), depending on the study and patient population.
- Both regimens offer similar efficacy overall, with no statistically significant difference in OS or progression-free survival in pooled analyses.
- Some data suggest NALIRIFOX may have a higher overall response rate, though this is not always statistically significant.
Safety and Side Effects
- NALIRIFOX tends to cause more severe diarrhea but fewer severe hematological (blood-related) side effects, such as low platelet counts.
- FOLFIRINOX is associated with more hematological toxicity but less severe diarrhea.
Summary of Regimens
NALIRIFOX- Median Overall Survival: 11.1 months
- Key Side Effects: More diarrhea, less hematologic toxicity
- Notes: May have a higher response rate
- Median Overall Survival: 9.1–11.7 months
- Key Side Effects: More hematologic toxicity, less diarrhea
- Notes: Well-established standard
Clinical Considerations
Both NALIRIFOX and FOLFIRINOX are considered preferred options for patients with stage 4 pancreatic cancer who are fit enough for aggressive chemotherapy.The choice between regimens should be individualized, taking into account the patient’s risk factors for gastrointestinal versus hematologic toxicity, other medical conditions, and personal preferences.
No direct head-to-head randomized clinical trial has definitively established one regimen as superior; most data come from indirect comparisons and real-world studies.
Potential Role of Fenbendazole and Ivermectin in Stage 4 Pancreatic Cancer
Given the limited success of conventional treatments, alternative therapies have attracted interest. Fenbendazole and ivermectin—antiparasitic drugs widely used in veterinary medicine—are being explored for their potential anti-cancer properties.- Several peer-reviewed studies and case reports suggest these agents may exert multiple anti-cancer mechanisms.
- Using SEER 5-year survival statistics as a benchmark (median survival ~3–6 months post-diagnosis) vs multiple cases reporting survival beyond one year with fenbendazole and ivermectin treatment.
Fenbendazole and Ivermectin in the Treatment of Stage 4 Pancreatic Cancer: A Compilation of Case Reports
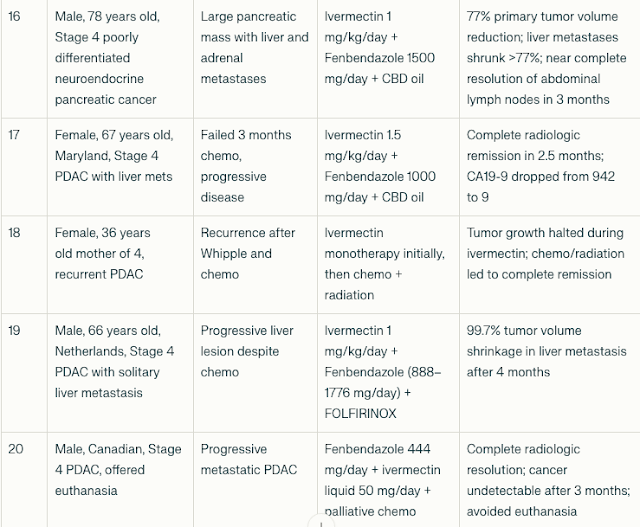
Patient Characteristics and Treatment Regimens
The cohort included 20 patients (age range 36–78 years; both sexes
represented) with advanced PDAC (Pancreatic Ductal AdenoCarcinoma),
many with liver, lung, or lymph node metastases. Several had failed
multiple lines of chemotherapy prior to initiation of fenbendazole and
ivermectin.
Fenbendazole doses ranged from 444 mg to 2000 mg daily; ivermectin doses ranged from 12 mg daily to 1.5 mg/kg/day. Some patients received adjunctive chemotherapy or radiation.
Tumor Marker and Imaging Responses
- CA19-9 reductions: Most patients experienced dramatic declines in CA19-9, with reductions ranging from 43% to >99%. For example, one 77-year-old patient’s CA19-9 dropped from 44,960 to 21 after fenbendazole and mebendazole therapy.
- Tumor shrinkage: Imaging showed significant tumor volume reductions, including a 99.7% shrinkage of a liver metastasis in one patient after 4 months of combined therapy.
- Remission: Several patients achieved no evidence of disease (NED) status or complete radiologic remission after treatment.
- Clinical improvement: Patients reported improved energy, weight gain, symptom relief, and extended survival beyond expected prognoses.
Abbreviations and Notes:
- PDAC: Pancreatic Ductal AdenoCarcinoma.
- CA19-9: Cancer antigen 19-9, a tumor marker for pancreatic cancer
- CEA: Carcinoembryonic antigen, another tumor marker
- NED: No evidence of disease
- CBD: Cannabidiol oil, used as adjunct in some cases
- FOLFIRINOX: Combination chemotherapy regimen (folinic acid, fluorouracil, irinotecan, oxaliplatin)
- Turbo Cancer: Term used to describe aggressive tumor growth post COVID-19 mRNA vaccination in some reports (hypothetical and unproven).
The compiled cases demonstrate consistent patterns of:
- Significant tumor marker reductions (CA19-9 and CEA)
- Radiological tumor shrinkage or complete response
- Clinical improvement in symptoms and quality of life
- Responses observed even in chemotherapy-resistant or advanced metastatic disease
- Use of fenbendazole and ivermectin as monotherapy or adjuncts to chemotherapy and radiation
How to Manage Stage IV Side Effects
Patients may also get treatment to control side effects, called supportive (palliative) care. This treatment focuses on comfort, quality of life and the patient’s total well-being. Supportive care can go with cancer-fighting treatments or be the focus of care.Proper nutrition and choosing the right foods can also help a patient better tolerate treatment, control side effects and improve quality of life.
The Pancreatic Cancer Action Network strongly recommends that patients have access to pancreatic enzymes and see a registered dietitian.
Why Is Stage IV Hard to Treat?
Pancreatic cancer is hard to treat at any stage. The more the cancer spreads, the more challenging treatment becomes.Surgery is the best option for long-term survival of pancreatic cancer. Because stage IV cancer has spread to different parts of the body, it cannot be removed by surgery.
Some patients also respond better to a certain treatment than others for unknown reasons. Stage IV patients may have to try different treatments before one works for them.
Also, a dense tissue layer, called the stroma, surrounds pancreatic tumors. This makes it hard for treatment to reach the tumor. Researchers are studying ways to get treatment through the stroma to make it more effective.
Questions to Ask Your Doctor when Diagnosed with Stage IV Pancreatic Cancer
- What treatment(s) do you recommend? Why?
- Are there any clinical trials available to me at this hospital? At other local hospitals?
- Do you offer biomarker testing or refer patients to the Pancreatic Cancer Action Network’s Know Your Tumor® precision medicine service to help find other treatment options?
- What medicine(s) will you prescribe to help control my side effects? Do these medicines cause other side effects?
- Do I need to change my diet?
- Is there a dietitian you recommend?
- Will I need to take pancreatic enzymes or vitamins? If so, how often?
- Should I make any lifestyle changes?
- What support programs are there for me and my family?
- Whom can I speak with about my financial or insurance concerns?
- Who can help me navigate the medical system? Is there an oncology social worker or patient navigator at this hospital?
Conclusion
Current best treatments for stage 4 pancreatic cancer involve aggressive multi-agent chemotherapy such as NALIRIFOX or FOLFIRINOX, with regimen choice tailored to patient tolerance and tumor genetics. Both regimens offer similar efficacy, with NALIRIFOX potentially providing a slight survival advantage but higher rates of diarrhea. FOLFIRINOX remains a well-established standard, particularly for patients at risk of gastrointestinal side effects.Combining chemotherapy with targeted or immunotherapy agents is an evolving and promising approach. Meanwhile, emerging evidence also suggests that repurposed drugs like fenbendazole and ivermectin could offer additional therapeutic options, warranting further clinical investigation.




.png)

.png)




.png)

Comments
Post a Comment