Bioshield Shows Sustained Early Promise in Recurrent GBM: Initial 100% Disease Control in Pilot Cohort, with Ongoing Trials - Dr. Patrick Soon-Shiong
Dr. Patrick Soon-Shiong, inventor of Abraxane and Executive Chairman of ImmunityBio, continues to highlight promising early results from the company's "Cancer BioShield™" platform across multiple indications, including recurrent glioblastoma (GBM)—one of oncology's most challenging cancers.
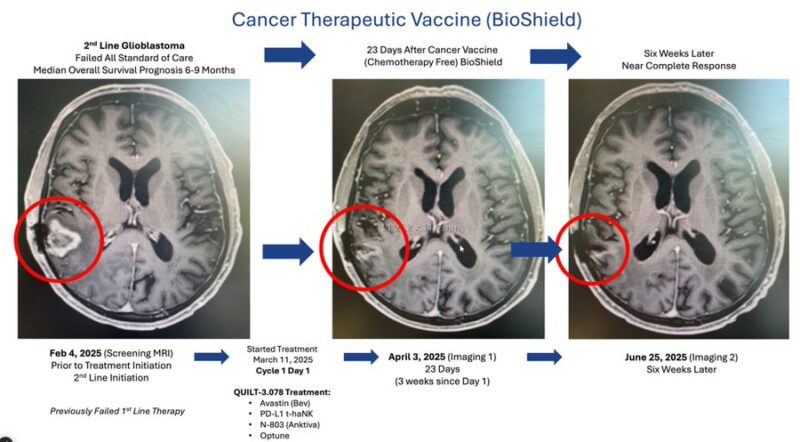
In an August 26, 2025 press release, ImmunityBio reported initial pilot data in recurrent GBM: All 5 treated patients achieved 100% disease control, with 2 near-complete responses and 3 objective responses overall. The chemo-free regimen combines Anktiva® (nogapendekin alfa inbakicept-pmln, an IL-15 superagonist), NK cell therapy, and supportive elements like Optune Gio® to protect and activate the immune system rather than deplete it.
Standard treatments often induce profound lymphopenia (~90% of cases), impairing NK and T cells and driving relapse. The BioShield approach reverses this by expanding killer cells.
At the Society for Neuro-Oncology (SNO) 2025 meeting, data linked tumor responses to NK cell expansion; randomized trials are now initiating.
Note on Cancer Vaccines: Therapeutic vaccines boost attack on existing tumors; preventive ones (e.g., HPV) activate immunity beforehand. Responses vary by patient-specific antigens.The GBM trial remains open (CSSIFM.org). Data preliminary (small cohort) but encouraging in a historically poor-prognosis disease.Recent Platform Validation: Durable Complete Remission in Waldenström's Macroglobulinemia (January 2026)On January 16, 2026, Dr. Soon-Shiong shared results (X.com) from a compassionate-use case in relapsed/refractory Waldenström's macroglobulinemia:
"We achieved complete remission in a patient who had failed all standards of care... bone marrow overtaken by >95% tumor. As a Hail Mary... we agreed to try our CAR-NK with Rituximab... after only 4 doses... complete response. Even after no further therapy... the patient REMAINS IN COMPLETE REMISSION AT 15 MONTHS AND ONGOING!"
This validates NK cell potency in "rescuing" failed therapies (paralleling Anktiva's prior rescues in bladder/lung). A recent Saudi FDA approval is described as breaking barriers toward paradigm shift. Dr. Soon-Shiong emphasized Anktiva as the only approved IL-15 superagonist, enabling next-generation in vivo CAR-T/NK therapy.Understanding ALC and Lymphopenia: Dr. Soon-Shiong's InsightsIn late 2025–early 2026 X posts, Dr. Soon-Shiong (@DrPatrick
) has stressed Absolute Lymphocyte Count (ALC) as a vital, often-overlooked marker on routine CBC tests:"Maintaining immune competence and ALC levels at least above 1.5 [1,500 cells/µL] matters so much for patients with cancer, with viral infections such as HPV and Covid, and even for longevity!!"
Until Anktiva, no therapy reliably reversed lymphopenia (ALC <1,000). Independent studies (Mayo, Harvard, UPenn) link low ALC to worse survival. Anktiva is FDA-approved for BCG-unresponsive bladder cancer with Expanded Access for lymphopenia reversal in solid tumors.ImmunityBio Company Review (Updated January 2026)ImmunityBio (NASDAQ: IBRX) is a clinical-stage biotech activating NK cells, T cells, and memory responses without heavy immunosuppression. Flagship product: Anktiva®, approved 2024–2025 across regions for bladder cancer; advancing broadly.Pipeline Highlights (as of Jan 2026):
- Recurrent GBM: Early 100% disease control (n=5); randomized trials starting.
- Waldenström's macroglobulinemia: Durable complete remission (15+ months ongoing) in relapsed/refractory patient using off-the-shelf CAR-NK + Rituximab.
- NSCLC: Positive lymphopenia reversal with checkpoint inhibitors; Saudi approval for metastatic combo.
- Bladder: Strong commercial traction; EU conditional authorization progressing.
- Other: Long COVID trial, prostate combos, CAR-NK platforms.
- Preliminary full-year net product revenue $113M (700% YoY growth from ~$14M in 2024).
- Driven by Anktiva launch momentum.
- Cash ~$243M (estimated end-2025); commercialization ramping.
Employee/Culture: Mixed reviews—strong innovation praise, some work-life concerns.Outlook: Rating 8.5/10 (up from prior). Scientific momentum accelerating (GBM signal, Waldenström's CR, approvals, NK validation) alongside commercial execution. Positions ImmunityBio as a leader in "Immunotherapy 2.0" via IL-15/NK platform. Risks: Burn rate, competition, regulatory timelines. Key near-term catalysts include full EU approval, lung data, and potential U.S. label expansions.
For latest updates: ImmunityBio IR, clinicaltrials.gov,
@ImmunityBio
/ @DrPatrick
on X.Labels: glioblastoma, waldenstrom, immunotherapy, anktiva, bioshield, nk-cells, car-nk, lymphopenia, alc, oncology
References:
Bioshield Sparks Early Promise in Recurrent GBM, With 2 Near-CRs and 100% Disease Control - Dr Patrick Soon-Shiong
Ivermectin and Mebendazole for Brain Cancer Success Stories: 115 Case Reports (December 2025)
.png)

.png)



.png)


Comments
Post a Comment