Iota Carrageenan (Betadine) Nasal Spray and Ivermectin: FLCCC I-MASK+ Protocol for COVID-19
If you are confused about the recommendations made by different professional groups for the COVID-19 pandemic, you've come to the right place. Before you continue to read this rather long article, let's start with the end in mind and begin with the conclusion that you may have been told. There is no early treatment for COVID-19? Most of the studies are small and are of low quality? We shall wait for bigger and better quality evidence before we can make formal recommendations?
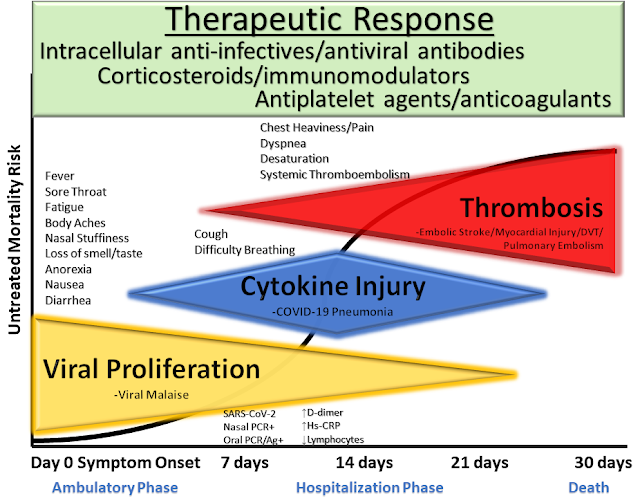 |
| McCullough et al. Reviews in Cardiovascular Medicine, 2020 |
In this article, we would like to cover 2 popular treatments i.e. Iota-Carrageenan and Ivermectin.
Iota-Carrageenan
Carragelose is a brand name of iota-carrageenan from Marinomed. Marinomed Biotech AG (VSE:MARI), an Austrian science-based biotech company with globally marketed therapeutics derived from innovative proprietary technology platforms, announced that Carragelose inactivates the new, rapidly spreading variants and SARS-CoV-2 wildtype with similar efficacy in vitro. The Company tested the three variants that currently mostly drive the COVID-19 pandemic, namely the so-called British or B.1.1.7, the South-African or B.1.351, and the Brazilian or P1 variant.[*] The data demonstrate that also with increasing prevalence of virus variants, the marketed OTC Carragelose-based lozenges, nasal and throat sprays will continue to effectively contribute to combatting the COVID-19 pandemic.
 |
| Iota-carrageenan nasal spray (Singapore) |
In recent in vitro tests, Marinomed included four lentiviruses differently pseudotyped with the spike protein of wild-type SARS-CoV-2 or one of the three variants B.1.1.7, B1.351 and P1, respectively. Carragelose was able to inactivate all four virus forms at concentrations below 5 µg/ml. This is clinically relevant for the use of Carragelose-containing products: The marketed nasal sprays have a Carragelose concentration of 1.2 mg / ml, a more than 200-fold higher dose as shown to be effective in vitro. The non-sulfated polymers HPMC and CMC were ineffective even at the highest concentrations tested.
In addition, two of the three SARS-CoV-2 variants (B1.1.7 and B1.351) were independently tested in Vero cell tissue culture in cooperation with the virological institute of the University Hospital Erlangen, Germany. Carragelose showed similar effectiveness against the SARS-CoV-2 wild type and the tested variants.
Dr. Prieschl-Grassauer continued: “We are very pleased to show that Carragelose is effective regardless of the actual SARS-CoV-2 variant. With the extensive discussions we are seeing around maintaining efficacy against a mutating virus, it is reassuring to know that Carragelose is a simple, safe, and effective means of supporting the prevention and treatment of COVID-19. With the data we have already seen against SARS-CoV-2 wild type, we are confident that this will hold true also for SARS-CoV-2 variants in the clinic.”
Marinomed’s lentivirus data show the ability of Carragelose to prevent the virus from attaching to the host cell. The infectious virus particles used in the cooperation with the virological institute of the University Hospital Erlangen mimic the effect of an actual infection, where the virus replicates in the host cells and then reinfects further cells, thereby spreading the infection in the body. Both are established and scientifically widely accepted models. Taken together, the data show how Carragelose can effectively inhibit SARS-CoV-2 variants in tissue culture. The cooperation partners plan to publish the data in a peer reviewed journal.
Check out the summary of evidence on Iota-Carrageenan versus COVID-19 from c19ic.com (constantly updated).
Ivermectin
Ivermectin is an anti-parasitic medication widely used in low- and middle-income countries to treat parasitic worm infections in adults and children. It’s been used for decades for this purpose by over 3.7 billion people, and is considered safe and effective. It has an increasing list of indications due to its antiviral and anti-inflammatory properties, and is included on the WHO’s Model List of Essential Medicines.
Ivermectin and Iota-Carrageenan
Letter to the Editor of the American Journal of Therapeutics reminding readers of the excellent RCT (Randomized Controlled Trial) results of an Ivermectin and Iota Carrageenan prophylaxis against frequency and severity of COVID infection.
FLCCC (Front Line COVID-19 Critical Care) I-MASK+ Protocol
About Carragelose®
Carragelose® is a sulfated polymer from red seaweed and is a unique, broadly active anti-viral compound. It is known as a gentle yet effective and safe prevention and treatment against respiratory infections. Several clinical and preclinical studies have shown that Carragelose® forms a layer on the mucosa wrapping entering viruses, thereby inactivating them, and preventing them from infecting cells. Increasing clinical evidence indicates that Carragelose® can also inactivate SARS-CoV-2.[MedRxiv],[Marinomed]
Marinomed is holder of the IP rights and has licensed Carragelose® for marketing in Europe, parts of Asia, Canada, and Australia. For a full list of Marinomed’s portfolio of Carragelose® containing nasal sprays and oral products, please visit https://www.carragelose.com/en/portfolio/launched-products, for a list of scientific publications on Carragelose®, please visit https://www.carragelose.com/en/publications.
Frequently Asked Questions
BETADINE® COLD DEFENSE works by an antiviral effect. It traps and eliminates viruses, thereby have effects on symptom relief. Our studies have looked at total symptoms and we have demonstrated that BETADINE® COLD DEFENSE will reduce the total symptoms such as runny nose, nasal congestion, sore throat, etc. when given as an early intervention for treatment of common cold and flu-like illness.
5. How long should BETADINE® COLD DEFENSE NASAL SPRAY be used? Is it well tolerated?
The recommendation is for up to 7 days.
The clinical trials have shown that BETADINE® COLD DEFENSE has a high tolerability, no nasal irritation, smell or taste.82,83,88 One of the key things is that the iota carrageenan in the spray is not absorbed by the body. It really is working physical means not by medicinal means. It is a medical device.
6. Is carrageenan contained in BETADINE® COLD DEFENSE NASAL SPRAY safe?
7. Can we use BETADINE® COLD DEFENSE NASAL SPRAY with Saline Nasal Sprays?
You can continue to use the saline spray to remove crusting and wash the nose. But you should always use the BETADINE® COLD DEFENSE after the saline spray, otherwise you are going to wash out the Cold Defense and reduce its efficacy. Always seek the advice of your physician with any questions you may have.
The concentration of BETADINE® Cold Defense for adult and paediatric is 1.2 mg/ml.86 Whilst the concentration is the same for both adults and kids, the spray mechanism is designed to deliver a smaller volume for kids noses.
9. What is the recommended dosage of BETADINE® COLD DEFENSE NASAL SPRAY?
The spray comes in two formulations: the adult spray and the kid spray. The adult spray is 1 spray into each nostril 3 times per day. For the kid spray, which delivers a smaller dose, 2-3 sprays into each nostril, 3 times a day.
10. What is the duration of the antiviral effect of BETADINE® COLD DEFENSE NASAL SPRAY after usage?
The antiviral effect in vivo, in human beings, the duration has not been measured, but we do know that when BETADINE® COLD DEFENSE is administered 3 times per days. It is effective in reducing severity of symptoms.
Where to buy Betadine Cold Defense Nasal Spray?
References:
- List of iota-carrageenan COVID-19 studies (constantly updated)
- Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review
- https://ph.betadine.global/en/ph/upper-respiratory-tract-infection-care/betadine-cold-defense-nasal-spray
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2923116/
- Amcyte Pharma Announces U.S. Launch of Nasitrol® Nasal Spray (Nov 2021)
- Combination of iota-carrageenan and xylitol as a potential nasal spray for COVID (Nov 2021)

.png)
.png)






Comments
Post a Comment