Hydroxychloroquine and Ivermectin Combined: A synergistic combination for COVID-19?
The number of options for the treatment of COVID-19 has increased drastically in recent months, thus making it complicated when it comes to choosing the right combination. In general, there are 3 broad categories of medical interventions:
- Prevention or Prophylaxis e.g. vaccine
- Early out-patient treatment
- Hospital treatment
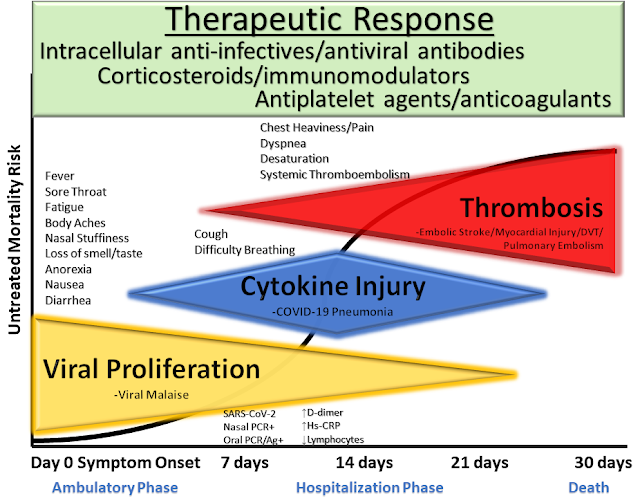 |
| McCullough et al. Reviews in Cardiovascular Medicine, 2020 |
All these treatments come with various technologies and jargons, thus could be overwhelming and confusing for you as a consumer. Generally, multiple treatments and strategies are used in combination to achieve the best possible outcome.
The medical community themselves are battling over ivermectin and hydroxychloroquine on whether they should be used to treat and prevent COVID-19. On one side are experts telling you that more research is needed before the treatment can be fully authorised and confirmed. On the other, are experts telling you that the potential benefits outweigh the risk and a 'wait and do nothing' position is not acceptable. Confused?
How do you deal with different expert groups dishing out conflicting guides? A common issue is that certain groups have pre-defined narrative that they would like to support. Therefore, only studies that support that pre-defined narrative are picked and cited as references. This is what we call as 'cherry-picking'. Cherry picking will naturally lead to a 'biased' and 'manipulated' decision. In order to get the truth out, scientific information needs to be analysed in a comprehensive, updated and non-biased manner.
In this article, we would like to cover 2 popular treatments i.e. Ivermectin and Hydroxychloroquine and the possible synergistic action if given together.
Ivermectin and COVID-19
Great article on where we stand on the COVID-19 treatment front debate - COVID19Crusher
Apr 26, 2021: The new FLCCC outpatient protocol (I-MASK+) with the addition of fluvoxamine and nasal/oral "sanitation". Fluvoxamine 50 mg twice daily for 10–14 days. Add to ivermectin if: 1) minimal response after 2 days of ivermectin; 2) in regions with more aggressive variants; 3) treatment started on or after day 5 of symptoms or in pulmonary phase; or 4) numerous co-morbidities/risk factors. Avoid if patient is already on an SSRI (Selective Serotonin Reuptake Inhibitor).
Apr 26, 2021: The new FLCCC hospital treatment protocol (MATH+) with the notable additions of Fluvoxamine and anti-androgen therapy (Dutasteride/Finasteride).
- Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19 by Kory et al., published on American Journal of Therapeutics.
- Dr. Satoshi Ōmura, co-author of the newly published paper, “Global trends in clinical studies of ivermectin in COVID-19” was one of the four researchers from Kitasato University in Tokyo, Japan who received the Nobel Prize in Physiology or Medicine in 2015 for their discovery of ivermectin. Global trends in clinical studies of ivermectin in COVID-19, published in the Japanese Journal of Antibiotics, March, 2021.
- A multi-centre randomised controlled study in Egypt (Elgazzar, Research Square) reported that the death rate was significantly lower in Ivermectin treated patients group (severe patients) vs non-Ivermectin group (2% vs 20%). 1,300 patients were included in this randomized controlled trial.
- This randomized controlled trial out of Iran (Hashim, pre-print) used Ivermectin and Doxycycline in mild, moderate, and severe hospitalized COVID-19 patients. No patients in the mild and moderate COVID-19 category died and 18% of the severe patients perished taking this medication combo. In the control group, no mild-moderate patients died, but 27% of the severe COVID patients died. The patients who also got Ivermectin had a shorter recovery.
- A randomized, double-blind, placebo-controlled, multicenter, phase 2 clinical trial at five hospitals (Iran) and 180 patients with mild to severe disease (Niaee, ResearchSquare, Nov 2020). Ivermectin as an adjunct reduced the rate of mortality, the duration of low oxygen saturation, and the duration of hospitalization.
- The ICON study in US, published in Chest, Oct 2020 reported that Ivermectin treatment was associated with lower death rate vs Control (13.3% vs 24.5%) during treatment of COVID-19, especially in patients with severe pulmonary involvement.
- A double-blinded randomised controlled study in Bangladesh (Mahmud et al) reported that the death rate was 0% (0/183) in the Ivermectin arm vs 1.67% (3/180) in the control arm in mild to moderate COVID-19 patients.
- The IDEA (Ivermectin, Dexamethasone, Enoxaparin and Aspirin) study from Argentina reported 1 death out of 167 patients studied. The patient that died was a severe COVID-19 patient that required ventilator support.
- The pre-AndroCoV trial from Brazil reported that early detection of COVID-19 followed by a pharmaceutical approach with different drug combinations (Azithromycin, Hydroxychloroquine, Nitazonide, Ivermectin) yielded irrefutable differences compared to non-treated controls in terms of clinical outcomes, ethically disallowing placebo-control randomized clinical trials in the early stage of COVID-19 due to the marked improvements.
- A retrospective study out of Bangladesh (Khan, Archivos de Bronconeumologia 2020). This retrospective study enrolled a total of 325 from April to June 2020. 248 adult COVID-19 patients were looked at in two groups, 115 received ivermectin plus standard care (SC), while 133 received only standard care (SC). This study showed that Ivermectin was efficient at rapidly clearing SARS-CoV-2 from nasal swabs (median 4 days). This was much shorter than in the COVID-19 patients receiving only SC (15 days) or receiving a combination of three antiviral drugs (7–12 days). In addition, fewer Ivermectin patients developed respiratory distress leading to ICU admission. In fact, with Ivermectin, there was a quick hospital discharge (median 9 days) in 114 out of 115 patients; the one remaining patient had been admitted with advanced disease.
Related: List of Doctors that will prescribe Ivermectin
Hydroxychloroquine and COVID-19
Hydroxychloroquine, developed in the 1950s from chloroquine, an old anti-malarial drug, is registered in around 60 countries under trade names such as Plaquenil, Quensyl and Plaquinol.

According to Steve Kirsch (published on TrialSiteNews):
Skeptics might argue the reason all the studies are positive is that journals are more likely to publish positive results than negative results. But in fact, there is a good argument that the bias is the reverse for HCQ, where negative studies are more likely to be published than positive studies. But in this case, those arguments don’t matter as the skeptics can’t point to a negative early treatment trial that has not been published so the debate is moot.
Now, let’s talk safety. HCQ is on the WHO list of essential medicines, i.e., one of the safest and most effective drugs in a health system.
Lupus patients are put on HCQ and remain on the drug for life. The drug was FDA-approved more than 65 years ago. In 2016, it was the 135th most-prescribed medication in the United States, with more than 4 million prescriptions. Dose escalation studies in lupus patients and in rheumatoid arthritis patients established that 800 mg per day for life and 1,200 mg per day for 6 weeks are extremely well-tolerated.
The WHO says HCQ is safe to take for autoimmune diseases or malaria. However, they admit that there is weak evidence supporting their contention that HCQ is unsafe to take for COVID. But the problem with this is 1) they admit that the certainty of the evidence is “low” to “very low” and 2) they don’t break it out by the disease phase. We are interested in early treatment, not late treatment. You can’t just lump all the studies into one analysis.
In order to see what is actually happening in early treatment patients when they take HCQ, I reached out to Brian Tyson and George Fareed, whose practice has used HCQ in treating more than 6,000 people of all ages with COVID. The risk of diarrhea and nausea/vomiting claimed by the WHO is both “very rare and very minimal.” In general, diarrhea is more likely to be caused by COVID than the drug.
Fareed said he has had “zero cardiac issues” with any patients. They have never had any reason to drop HCQ from their treatment protocol and I don’t know of any physician in the US who has a lower rate of hospitalization for COVID than Tyson and Fareed. If the WHO is right, then how do they explain this anomaly? Tyson and Fareed certainly didn’t get lucky on 6,000 patients and the average age of their patients is 60 years old.
So the bottom line so far is 29 studies all positive, and real-world evidence on thousands of cases is also consistent with the studies. Our hypothesis that the drug is effective is consistent with the data. But the WHO and NIH say we should not use this drug, yet have no plausible explanation for the consistently positive data.
Some scientists will cite the HCQ analysis published in Nature which definitively shows that HCQ is harmful. But that was a meta analysis, which heavily weighted studies of high dose HCQ given to very late stage hospitalized patients. No early treatment outpatient trials were included. The paper says “Findings have unclear generalizability to outpatients, children, pregnant women, and people with comorbidities.” I agree!

Here’s a simple analogy as to why drug timing makes a huge difference: a small bucket of water works great if the fire is small (early stage). After the house burns down, the same bucket of water will do nothing to repair the damage, even if we increase the amount of water, and will probably further damage any remains.
Other scientists might reference the fact that the FDA revoked the EUA on HCQ, but the revocation was based on studies on hospitalized patients, not outpatients. So that argument doesn’t hold water.
In short, HCQ is both effective and safe for early treatment at dosages of 600mg per day and more. If anyone tells you otherwise, please ask them for both clinical studies and real-world evidence to back up their claim. At that point, they will say that they don’t have time to talk to you and walk away. This happens to me all the time. It’s frustrating.
Source: Page 16 of FLCCC Alliance – COVID-19 Management Protocol (version May 25, 2021)
Ivermectin and Hydroxychloroquine
- Hydroxychloroquine (HCQ) 200mg 2 times a day for 5-7 days
- Ivermectin 0.4 mg/kg/day for 5-7 days


.png)
.png)



.jpg)

Comments
Post a Comment