Methodology
Why evidence-based? A proven method trumps an unproven one.
We've scoured the internet, unearthed the best references, and reviewed
over 1,000 studies so you don't have to. When interpreting and filtering
scientific research, it's crucial to consider the hierarchy and quality of
evidence. Not all evidence is created equal. So, what is good
evidence?
Cell culture findings carry less weight than results from studies
conducted on mice. Similarly, conclusions from mouse studies are surpassed
by findings from human studies.
Case studies and preliminary results from small-scale human trials
hold less significance than outcomes from umbrella reviews, systematic
reviews and meta-analysis*, randomised controlled trials (RCTs), and
more extensive, long-term human trials.
*A systematic review is a review that collects, critically appraises,
and synthesises all the available evidence to answer a specifically
formulated research question. A meta-analysis, on the other hand, is a
statistical method that is used to pool results from various
independent studies, to generate an overall estimate of the studied
phenomenon.
It would be impossible to review all the studies on the internet;
rather, we have focused on, curated and evaluated the information that
appear to have the greatest clinical utility.
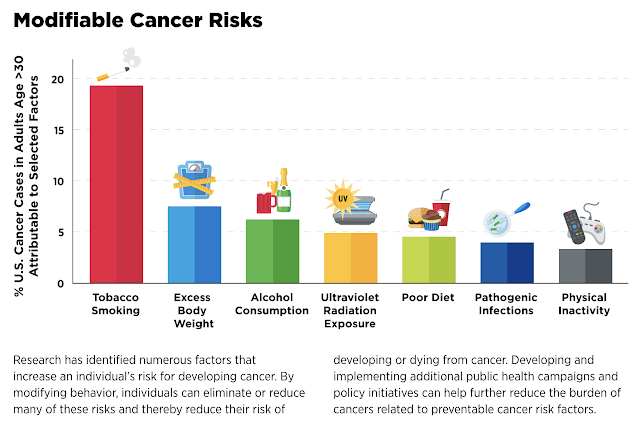
Causes of Cancer
Why is it important to know? By knowing the root causes, we will
better understand why we are using certain strategies to prevent
cancer.
As depicted below, most of the root causes of cancer are diet and
lifestyle related.
|
|
AACR Cancer Progress Report 2023
|
Therefore, it makes sense to improve your diet and lifestyle in
order to prevent or to treat early cancer.
From research point of view, cancer is daunting in the breadth and
scope of its diversity, spanning genetics, cell and tissue biology,
pathology, and response to therapy.
The Hallmarks of Cancer were proposed as a set of functional
capabilities gained by human cells as they transform from normal cells
to cancer states.
Biological Changes and Acquired Capabilities (
Cell 2000):
- sustaining proliferative signaling,
- evading growth suppressors,
- resisting cell death,
- enabling replicative immortality,
- inducing angiogenesis, and
- activating invasion and metastasis.
- deregulating cellular energetics
- avoiding immune destruction
- Genome instability and mutation
- Tumor promoting inflammation
- unlocking phenotypic plasticity,
- non-mutational epigenetic reprogramming,
- polymorphic microbiomes
- senescent cells.
The ACS 2022 Guideline for Cancer Survivors
General recommendations for cancer survivors
-
Nutritional assessment and counseling should begin as soon as
possible after diagnosis, with the goal of preventing or resolving
nutrient deficiencies, preserving muscle mass, and managing side
effects of treatments that may adversely affect nutritional
status.
-
Physical activity assessment and counseling should begin as soon
as possible after diagnosis, with the goal of helping patients
prepare for treatments, tolerate and respond to treatments, and
manage some cancerrelated symptoms and treatment-related side
effects.
Recommendations to improve long-term health and increase the
likelihood of survival
-
Avoid obesity and maintain or increase muscle mass through diet
and physical activity. •
-
Engage in regular physical activity, with consideration of type of
cancer, patient health, treatment modalities, and symptoms and
side effects.
-
Follow a healthy eating pattern that meets nutrient needs and is
consistent with recommendations to prevent chronic disease.
-
Follow the general advice of the American Cancer Society Guideline
for Diet and Physical Activity for Cancer Prevention to reduce
risk of a new cancer.
In 2020, the
American Cancer Society (ACS) published diet and physical activity guidelines for
cancer prevention. Nutrition and physical activity recommendations
established by the ACS for the primary prevention of cancer are
broadly relevant to survivors undergoing and immediately after
cancer treatment.
Diet and Cancer
If oral intake does not support adequate nutrient intake to meet
energy expenditure, the Recommended Dietary Allowance for vitamins and
minerals, and >1 g of protein per kilogram of body weight per day,
then the use of an oral nutritional supplement should be implemented.
If intake remains insufficient, consideration should be given to
additional nutrition support strategies, such as an enteral nutrition
tube feeding regimen; and, if enteral nutrition support is
contraindicated, parenteral nutrition support could be considered to
meet nutritional needs.
The National Comprehensive Cancer Network (NCCN) and the American
Society for Clinical Oncology (ASCO) recently published guidelines for
cancer survivors and their clinicians outlining diet, nutrition, and
physical activity recommendations.
Highlights include:
-
Recommendations to eat a healthy diet pattern, with adequate
macronutrient and micronutrient content from both animal-based and
plant-based food options but with a preference to plant-based diet
patterns;
-
Caution regarding the overuse and misuse of dietary supplements
during and after treatment;
-
Adherence to food safety procedures to avoid foodborne illnesses;
and
- Being as physically active as possible.
Quit smoking
The recommendation to quit smoking, established by the ACS for the
primary prevention of cancer are also relevant to survivors undergoing
and immediately after cancer treatment.
Cigarette smoking topped the charts as the leading risk factor,
contributing to nearly 20 percent of all cancer cases and close to 30
percent of cancer deaths. Smoking comprised 56 percent of potentially
preventable cancers in men and almost 40 percent of those in women.
(
Journal of the American Cancer Society 2024)

|
|
Journal of the American Cancer Society 2024
|
If you are a non smoker, then your risk of cancer will be reduced.
Smoking is by far the leading risk factor for lung cancer. In the early
20th century, lung cancer was much less common than some other types of
cancer. But this has changed once manufactured cigarette became readily
available and more people began smoking.
Avoid tobacco products altogether, including cigarettes, cigars, and
smokeless tobacco. About 80% of lung cancer deaths are thought to result from smoking.
The risk for lung cancer among smokers is many times higher than among
non-smokers. The longer you smoke and the more packs a day you smoke,
the greater your risk.
On top of that, you should also try to cut down on your visits to
places where people tend to smoke e.g. pubs etc. Passive smoking is
just as bad.
Cancer and Physical Activity
Most studies support that exercise is generally safe for individuals
undergoing cancer treatment. However, because most of these studies
are randomized controlled trials that may include healthier
participants than the general population of patients with cancer, it
is important for patients who have cancer to seek medical evaluation
to inform their individual exercise program during
treatment.
This type of guidance is valuable in creating a safe and effective
fitness plan for patients who have cancer with appropriate and
tailored modifications related to specific cancer diagnosis or
treatment-related issues, such as breast cancer-related lymphedema.
Individuals undergoing cancer treatment are encouraged to be active
members of their nutrition and physical activity care planning team.
Interventions during and immediately after treatment should be
individualized and realistic and should have scientific support.
The Metabolic Approach to Treating Cancer
Although mitochondrial replacement therapy could, in principle, restore a
more normal energy metabolism and differentiated state to tumor cells, it is
unlikely that this therapeutic approach would be available in the
foreseeable future.
However, if cancer is primarily a disease of energy metabolism, then
rational approaches to cancer management can be found in therapies that
specifically target energy metabolism. The goal of metabolic adjunctive
treatments is to “starve the cancer cell” by modulating energy pathways that
are important to the survival of cancer cells and thereby reduce cancer
growth and cancer metastases (the cause of death in over 90% of cancer
patients).
An approach to cancer treatment is emerging with research showing impressive
results from the use of metabolically targeted drug cocktails alongside
conventional chemotherapy. The metabolic protocol is designed to work
primarily by restricting the overall ability of cancer cells to take up and
use (i.e., ‘metabolize’) energy. By starving cancer cells of energy
substrates, metabolic interventions may reduce the capacity of cancer cells
to defend themselves against chemotherapy and radiation.
The metabolic protocol may also act on the many dysregulated signaling
pathways within cancer cells helping to enable apoptosis, or “programmed
cell death,” allowing chemotherapy and radiation to kill cancer cells more
effectively. The most important and central approach to the metabolic
treatment of cancers is dietary calorie (glucose) restriction. This is
supplemented with pharmacologic and nutraceutical compounds that target
specific cancer pathways and interventions that restore “normal” anticancer
immunity and prevent metastases.
It is important to emphasize that there is no single “magic bullet” and that
multiple interventions act synergistically and simultaneously to promote
cancer cell death. The combination of dietary interventions together with
multiple repurposed drugs/nutraceuticals that act synergistically is
strongly recommended.
However, similar to the work of Jane McLelland, we suggest a more extensive
and targeted list of pharmacologic and nutraceutical compounds combined with
glucose restriction and a ketogenic diet.
The metabolic approach to cancer should be considered as adjunctive to more
“traditional” approaches to cancer treatment. The metabolic treatments will
likely act synergistically with the more traditional approaches, thereby
increasing tumor response rate, limiting the toxicities of standard
chemotherapy, limiting the risk of metastasis, and leading to an improvement
in overall quality of life. This combined approach will allow for reduced
dosages of standard chemotherapeutic agents, drastically reducing their
toxicity.
Dietary Caloric Restriction, The Ketogenic Diet, and “Real” Food
Numerous studies show that dietary energy restriction is a general metabolic
therapy that naturally lowers circulating glucose levels and significantly
reduces the growth and progression of numerous tumor types, including
cancers of the breast, brain, colon, pancreas, lung, and prostate.
An impressive body of evidence indicates that dietary energy restriction can
retard the growth rate of many tumors regardless of the specific genetic
defects expressed within the tumor. Hyperglycemia with high insulin levels
is associated with tumor recurrence.
Sugar sweetened beverages are associated with an increased risk of cancer.
Both experimental and clinical data suggest that fructose, particularly
fructose-corn syrup, is more carcinogenic than glucose.
As demonstrated by Dr. Otto Warburg, almost all cancer cells are dependent
on glucose as a metabolic fuel via aerobic glycolysis, with hyperglycemia
being a potent promotor of tumor cell proliferation and associated with poor
survival.
Although the mechanisms responsible for the caloric-restriction-mediated
reduction in tumorigenesis have not been unequivocally identified, they may
involve caloric-restriction-induced epigenetic changes as well as changes in
growth signals and in the sirtuin pathway.
Insulin resistance plays a major role in the initiation and propagation of
cancer. Reversing insulin resistance is therefore a major goal in patients
with cancer. Dietary energy restriction specifically targets several
cancer hallmarks (see above, under 'causes of cancer') including cell
proliferation, evasion of apoptosis, and angiogenesis.
Dietary energy restriction targets inflammation and the signaling pathways
involved with driving tumor angiogenesis. Indeed, calorie restriction is
considered a simple and effective therapy for targeting tumor angiogenesis
and inflammation.
Calorie restriction results in the downregulation of multiple genes and
metabolic pathways regulating glycolysis. Besides lowering circulating
glucose levels, dietary energy restriction elevates circulating levels of
fatty acids and ketone bodies.
Fats, and especially ketones, can replace glucose as a primary metabolic
fuel under calorie restriction. This is a conserved physiological adaptation
that evolved to spare protein during periods of starvation. Many tumors,
however, have abnormalities in the genes and enzymes needed to metabolize
ketone bodies for energy. Elevation in ketone bodies is well known to be
able to suppress blood glucose levels and glycolysis, which are major
drivers of tumor growth.
A transition from carbohydrates to ketones for energy is a simple way to
target energy metabolism in glycolysis-dependent tumor cells while enhancing
the metabolic efficiency of normal cells. Metabolism of ketone bodies and
fatty acids for energy requires inner mitochondrial membrane integrity and
efficient respiration, which tumor cells largely lack.
Under fasting conditions, ketone bodies are produced in the liver from fatty
acids as the main source of brain energy. Ketone bodies bypass the
glycolytic pathway in the cytoplasm and are metabolized directly to acetyl
CoA in the mitochondria.
The ketogenic diet is a high-fat, low-carbohydrate diet with adequate
protein and calories originally developed in the 1920s as a treatment for
intractable epilepsy. The traditional ketogenic diet is a 4:1 formulation of
fat content to carbohydrate plus protein. A classic 4:1 ketogenic diet
delivers 90% of its calories from fat, 8% from protein and only 2% from
carbohydrate.
Ketogenic diets of the 1920s and 1930s were extremely bland and restrictive
diets and, therefore, prone to noncompliance. In recent years, alternative
keto-genic protocols have emerged, making adherence to the diet much easier.
Alternatives to the traditional keto-genic diet includes a medium-chain
triglyceride (MCT)-based ketogenic diet and the Atkins diet. Compared to
long-chain triglycerides, MCTs are more rapidly absorbed into the
bloodstream and oxidized for energy because of their ability to passively
diffuse through membranes. Another characteristic of MCTs is their unique
ability to promote ketone body synthesis in the liver. Thus, adding MCTs to
a ketogenic diet would allow significantly more carbohydrates to be
included.
A ketogenic diet has tumor growth-limiting effects, protects healthy cells
from damage by chemotherapy or radiation, accelerates chemotherapeutic
toxicity toward cancer cells, and lowers inflammation. Altered availability
of glucose and induction of ketosis influence all the classically defined
hallmarks of cancer.
Weber et al demonstrated that ketogenic diets slow melanoma growth in vivo
regardless of tumor genetics and metabolic plasticity. Moreover, ketogenic
diets simultaneously affected multiple metabolic pathways to create an
unfavorable environment for melanoma cell proliferation. In glioma cancer
models a ketogenic diet has been shown to reduce angiogenesis, inflammation,
peri-tumoral edema, migration and invasion.
The ketogenic diet may work in part as an immune adjuvant, boosting
tumor-reactive immune responses in the microenvironment by alleviating
immune suppression. A meta-analysis on the use of ketogenic diet in animal
models demonstrated significantly prolonged survival time and reduced tumor
weight and tumor volume. The ketogenic diet was effective across a broad
range of cancers.
The ketogenic diet is an effective adjuvant to radiation therapy for the
treatment of malignant glioma.(293) Ketone bodies have been shown to inhibit
histone deacetylases and may decrease tumor growth. In addition, the ketone
bodyβ-hydroxybutyrate acts as an endogenous histone deacetylase inhibitor,
resulting in downstream signaling that protects against oxidative stress.
The randomized controlled trial by Chi et al describes how adhering to a
caloric-restricted diet for 6 months can have therapeutic benefits in
slowing the growth of prostate cancer. The men in the control group
were instructed to avoid any dietary changes, whereas the men in the
calorie-restricted group were coached by a dietician to restrict dietary
carbohydrates to <20 grams/day. The authors found that elevated levels of
serum ketone bodies at both 3 and 6 months were associated with
significantly longer prostate cancer antigen doubling time (p < 0.0001),
which is a marker of prostate cancer growth rate.
Similarly, in a post hoc exploratory analysis of the CAPS2 randomized study
the PSA doubling time was significantly longer in the low carbohydrate diet
versus control diet (28 vs. 13 months, P = 0.021) arms. These findings
support the concept that elevations in ketone bodies are associated with
reduced tumor growth.
In a randomized trial in women with endometrial or ovarian a ketogenic diet
was associated with a significant improvement in physical function scores
with less fatigue. In this study the ketogenic diet resulted in the
selective loss of fat mass, retention of lean mass with lower fasting serum
insulin levels.
In a RCT Khodabakshi et al determined the feasibility, safety, and
beneficial effects of an MCT-based Ketogenic diet in patients with locally
advanced or metastatic breast cancer and planned chemotherapy. Compared to
the control group, fasting blood glucose, BMI, body weight, and fat% were
significantly decreased in intervention group (P < 0.001). Overall
survival in neoadjuvant patients was higher in the ketogenic group compared
to the control (P = 0.04).
A ketogenic diet following completed courses of chemotherapy and
radiotherapy was further reported to be associated with long-term survival
in a patient with metastatic non-small cell lung cancer. “Long-term”
survival has been reported in patients with glioblastoma on a ketogenic
diet. Furthermore, evidence shows that therapeutic ketosis can act
synergistically with conventional chemotherapeutic drugs, irradiation, and
surgery to enhance cancer management, thus improving both progression-free
and overall survival.
In addition, it is highly likely that therapeutic ketosis acts
synergistically with the repurposed anticancer drugs reviewed in this
document. Therapeutic ketosis requires a blood glucose < 90 mg/dl and a
blood ketone > 2 mmol/l, aiming for a glucose-ketone index (GKI) <
2.
See the GKI calculator in the section on caloric restriction. There are no
known drugs that can simultaneously target as many tumor-associated
signaling pathways as can calorie restriction. Hence, energy restriction can
be a cost-effective adjuvant therapy to traditional chemo- or radiation
therapies, which are more toxic, costly, and generally less focused in their
therapeutic action than dietary energy restriction. It should be noted that
the medium-chain fatty acids that are present during the consumption of a
ketogenic diet directly inhibit glutamate receptors.
Shukla et al observed reduced glycolytic flux in tumor cells upon treatment
with ketone bodies. Ketone bodies also diminished glutamine uptake, overall
ATP content, and survival in multiple pancreatic cancer cell lines, while
inducing apoptosis.
According to Dr. Seyfried: “Most human metastatic cancers have multiple
characteristics of macrophages. We found that neoplastic cells with
macrophage characteristics are heavily dependent on glutamine for growth. We
have not yet found any tumor cell that can survive for very long under
prolonged restriction of glucose and glutamine. Furthermore, we have not yet
found any fatty acid or ketone body that can replace either glucose or
glutamine as a growth metabolite. It, therefore, becomes essential to
simultaneously restrict both glucose and glutamine while placing the person
in nutritional ketosis for successful cancer management.”
Although dietary energy restriction and anti-glycolytic cancer drugs will
have therapeutic efficacy against many tumors that depend largely on
glycolysis and glucose for growth, these therapeutic approaches could be
less effective against those tumor cells that depend more heavily on
glutamine than on glucose for energy. Glutamine is a major energy metabolite
for many tumor cells and especially for cells of hematopoietic or myeloid
lineage.
Green tea polyphenol (EGCG) targets glutamine metabolism by inhibiting
glutamate dehydrogenase activity under low glucose conditions (see section
below). In addition, mebendazole, curcumin and resveratrol inhibit
glutaminolysis. Glioblastoma, breast cancer, pancreatic cancer, lung cancer,
prostate cancer, and lymphoma may depend on glutamine as a source of
energy.
Real Food: The Banting Diet
Patients are strongly recommended to eat “real food” and not processed food.
If it looks like food, it is likely food. If it comes in a box or carton,
has a food label, and/or a long list of chemicals and additives with long
and complex names it is not food. A high proportion of the population
(60-80%) eating a Western diet are addicted to processed food.
Processed food addiction is a recognized “substance use disorder” (SUD) and
should be treated as such. Animal experiments demonstrate that sugar and
fructose are more addictive than cocaine and heroin and that carbohydrate
addicts demonstrated many of the behaviors of those with an SUD.
Results from the NutriNet-Santé prospective cohort study demonstrated that a
10% increase in the proportion of ultra-processed foods in the diet was
associated with a significant increase of greater than 10% in risks of
overall and breast cancer.
The EPIC Cohort study investigated the association between dietary intake
according to amount of food processing and risk of cancer at 25 anatomical
sites using data from the European Prospective Investigation into Cancer and
Nutrition (EPIC) study. In this study, in a multivariate model, substitution
of 10% of processed foods with an equal amount of minimally processed foods
was associated with reduced risk of overall cancer (hazard ratio 0·96, 95%
CI 0·95-0·97), head and neck cancers (0·80, 0·75-0·85), oesophageal squamous
cell carcinoma (0·57, 0·51-0·64), colon cancer (0·88, 0·85-0·92), rectal
cancer (0·90, 0·85-0·94), hepatocellular carcinoma (0·77, 0·68-0·87), and
postmenopausal breast cancer (0·93, 0·90-0·97).
A low carbohydrate-high fat (LCHF) dietary pattern is especially important
for patients with cancer. As already discussed, a low carbohydrate ketogenic
diet is essential to control blood glucose levels. Furthermore, a real food
diet high in both soluble and insoluble fiber and fermented foods is
critical to normalize the microbiome. Alterations in the microbiome play an
important role in both tumorigenesis and tumor propagation. Altered gut
microbiota is associated with resistance to chemotherapeutic drugs while
restoration of a normal microbiome improves the response to the anticancer
drugs.
Antibiotics cause severe dysbiosis which is associated with an increased
risk of cancer and reduced response to chemotherapy. The Banting Diet comes
close to meeting the criteria of the ideal real-food diet. William Banting
(1796-1878), a Victorian undertaker, is regarded as the father of the low
carbohydrate diet. In 1863, Banting wrote a booklet called Letter on
Corpulence, Address to the Public, which contained the particular plan for
the diet he followed.
It was written as an open letter in the form of a personal testimonial.
Banting described all of his unsuccessful fasts, diets, spa visits, and
exercise regimens all of which had been advised by various medical experts.
He then described the dietary change that finally had worked for him,
following the advice of another medical expert. "My kind and valued medical
adviser is not a doctor for obesity, but stands on the pinnacle of fame in
the treatment of another malady, which, as he well knows, is frequently
induced by [corpulence]."
His own diet consisted of meat, greens, fruits, and dry wine. The emphasis
was on avoiding sugar, saccharine matter, starch, beer, and milk. Banting's
pamphlet was popular for years to come and would be used as a model for
modern diets. The Banting diet consists mainly of animal protein (including
poultry, eggs, and fish), saturated animal fats (including lard, duck fat,
and butter), coconut oil, olive oil, and macadamia oil, some cheeses and
dairy products, some nuts and seeds, fresh vegetables grown mainly above the
ground and a few berries.
The Banting diet excludes all processed package foods and fast foods. It
also excludes all foods with sugar, fructose, and maltose as well as grain
products (wheat, barley, oats, rye) and soy products. Soy products are
genetically modified, toxic non-foods. Replace all seed oils (canola,
sunflower, safflower, cottonseed, soy) with healthy saturated fats, extra
virgin olive oil and virgin coconut oil are freely encouraged. High fat
dairy products are suggested but not skimmed or fat-free dairy products.
Intermittent Fasting, Autophagy, and Cancer
Fasting has a profound effect on promoting immune system homeostasis,
improving mitochondrial health, and increasing stem cell production. Fasting
stimulates the clearing of damaged mitochondria (mitophagy), misfolded and
foreign proteins, and damaged cells (autophagy). Intermittent
fasting/time-restricted eating is the single most effective method to
activate autophagy.
However, the role of intermittent fasting and autophagy in cancer is
complex. The 2016 Nobel Prize in Physiology or Medicine was awarded to
Yoshinori Ohsumi for his initial elucidation of the morphological and
molecular mechanisms of autophagy in the 1990s. Macroautophagy (herein
referred to as autophagy) is a conserved lysosomal degradation pathway for
the intracellular recycling of macromolecules and clearance of damaged
organelles and misfolded proteins to ensure cellular homeostasis.
During autophagy, cytoplasmic constituents (damaged proteins, misfolded
proteins, foreign proteins) are engulfed within double-membrane vesicles
called autophagosomes, which subsequently fuse with lysosomes to form
autolysosomes, where the cargo is degraded or recycled.
Autophagy occurs at basal levels under physiological conditions and can be
upregulated in response to stressful stimuli such as hypoxia, nutritional
deprivation, DNA damage, and cytotoxic agents. The molecular machinery that
mediates the autophagic process is evolutionarily conserved in higher
eukaryotes and regulated by specific genes (ATG genes), which were initially
characterized in yeast.
The process of macroautophagy can also lead to cell death or “autophagic
cell death,” as a result of the accumulation of autophagosomes and
autolysosomes in the cytoplasm. The effects of fasting, autophagy and cancer
are still under study, but many researchers propose that intermittent
fasting could help with the treatment and eradication of tumors and cancer
cells.
Intermittent fasting/time-restricted eating is the most effective therapy
for the treatment of insulin resistance, metabolic syndrome, and type II
diabetes. Intermittent fasting has additional benefits in prolonging health
span, alleviating the symptoms/curing many chronic diseases, as well as
preventing cardiovascular disease, Alzheimer’s disease and cancer. To gain
the maximum benefits of intermittent fasting, it is thought that feeding
times should be scheduled to align with circadian rhythms and activities so
that timely nutrient metabolism favors healthy physiology.
The metabolic effects of intermittent fasting are numerous and include
increasing insulin sensitivity, decreasing blood glucose levels, decreasing
insulin levels, decreasing insulin-like growth factor, activating the
sirtuin pathway, and activating autophagy. Intermittent fasting is the most
effective means of activating autophagy and accounts for many of its
beneficial effects. These effects likely explain the benefits of
intermittent fasting in patients with cancer.
There has been some concern that while autophagy may play an important role
in preventing the development of cancer, it may paradoxically promote cancer
cell proliferation. Once a tumor is established, the main function of
autophagy is to provide a means to cope with cellular stressors, including
hypoxia, nutritional and growth factor deprivation, and damaging stimuli,
thus allowing tumor adaptation, proliferation, survival, and dissemination.
While autophagy may theoretically promote cancer cell proliferation multiple
studies have demonstrated that autophagy leads to cancer cell death.
Almost all the repurposed drugs listed in this monograph have been
demonstrated to enhance tumor cell death by activating the autophagy
pathway. Limited rodent studies and human studies have evaluated the
independent effects of intermittent fasting/time restricted eating in
modulating cancer progression. In a study of a high-fat driven,
postmenopausal breast cancer mouse model, intermittent fasting markedly
inhibited tumor initiation, progression, and metastasis compared with mice
fed ad libitum in the absence of calorie restriction or weight loss.
This beneficial effect of intermittent feeding was probably mediated, at
least in part, by reduced insulin signaling because systemic insulin
infusion through implanted pumps reversed the intermittent fasting-mediated
cancerprotective actions. Additional animal models have demonstrated the
benefit of intermittent fasting on cancer progression.
Fasting seems to improve the response to chemotherapy by several mechanisms
including:
-
Enhances DNA repair in normal cells but not in malignant cells
-
Improves autophagy mechanisms as a protection against damage to
organelles
-
Promotes apoptosis by both increasing tumor cell susceptibility to
apoptotic stimuli, and averting apoptosis-mediated damage to normal
cells
-
Decreases regulatory T cells and enhances stimulation of CD8 cells.
Interestingly, fasting in combination with cytotoxic agents elicited
differential responses in normal and cancer cells, a phenomenon known as
differential stress resistance (DSR). For DSR, normal cells prioritize
maintenance pathways and inactivate growth factor signaling when nutrients
are absent. In contrast, cancer cells, due to oncogene activation, do not
inhibit stress resistance pathways, thus becoming vulnerable to cytotoxic
treatment.
The role of intermittent fasting and the enhancement of autophagy in
patients with cancer is complex. While animal models demonstrate a benefit
of intermittent fasting in several tumor models, clinical data in humans is
limited. While time-restricted feeding (intermittent fasting) may
theoretically promote cancer cell proliferation, this concept has not been
observed in patients with cancer. Furthermore, more prolonged fasting of
24-96 hours has been well tolerated in patients with cancer and appears to
improve quality of life and disease symptoms.
This data suggests that the approach to intermittent fasting should be
individualized in patients with cancer according to each patient's response.
Data from small trials in humans suggest that many types of intermittent
fasting regimens positively affect risk factors for poor breast cancer
outcomes, such as glucoregulation, inflammation, obesity, and sleep.
Experimental animal models and human data support the hypothesis that a
prolonged nightly fasting interval (time restricted eating) could reduce
cancer risk and improve cancer outcomes.
Marinac et al investigated whether the duration of nightly fasting predicted
recurrence and mortality among women with early-stage breast cancer. Data
were collected from 2413 women with breast cancer but without diabetes
mellitus who were aged 27 to 70 years at diagnosis and participated in the
prospective Women’s Healthy Eating and Living study. Nightly fasting
duration was estimated from 24-hour dietary recalls collected at baseline,
year 1, and year 4. The mean fasting duration was 12.5 ± 1.7 hours per
night. In repeated-measures Cox proportional hazards regression models,
fasting less than 13 hours per night was associated with an increase in the
risk of breast cancer recurrence compared with fasting 13 or more hours per
night (HR 1.36; 95% CI, 1.05-1.76).
Metabolic and Lifestyle Interventions for Cancer Management
1. Glucose Management and the Ketogenic Diet
A carbohydrate-restricted diet (less than 25 g of carbs per day) that is
high in saturated fat and Omega-3 fatty acids (ketogenic diet) is suggested.
Avoid all processed food. Contrary to current dogma, saturated fatty acids
are “healthy,” but avoid processed omega-6 vegetable oils or linoleic acid
(see below).
Avoid foods that are high on the glycemic index and follow the “hacks” to
flatten the blood glucose curve (see below). A continuous glucose monitor
(CGM) is essential to track changes in blood glucose levels. Patients must
keep accurate records to identify (and avoid) any food that might spike
blood glucose. Target a baseline blood glucose of 60-80 mg/dl (3.3 – 4.4
mmol/l) and a postprandial (after a meal) glucose of less than 120 mg/dl
(6.6 mmol/l). The ideal is a flat blood glucose curve; the blood glucose
should not increase by more than 20 mg/dl after a meal. In addition, a blood
ketone meter (blood level of beta-hydroxybutyrate) is recommended to confirm
that the patient has entered ketosis (normal level < 0.5 mmol/l).
Ideally, the blood ketone level should be over 2 mmol/l. The optimal
therapeutic range is between 3 and 5 mmol/l. It is important to track
changes in blood glucose and ketones with both fasting and exercise.
Therapeutic ketosis requires a blood glucose < 90 mg/dl and a blood
ketone > 2 mmol/l, aiming for a GKI of < 2.
The Glycemic Index
The glycemic index (GI) is a value assigned to foods based on how quickly
those foods cause increases in blood glucose levels and how high they spike.
The glycemic index ranks food on a scale from 0 to 100. Pure glucose is
arbitrarily given a value of 100, which represents the relative rise in the
blood glucose level after two hours (see Figure 7). The GI of a specific
food depends primarily on the quantity and type of carbohydrate it contains
(see Table 4). Foods that are low on the GI scale tend to release glucose
slowly and steadily. Foods that are high on the glycemic index release
glucose rapidly. It should be noted that the glycemic index varies among
individuals. (360, 361) A continuous glucose monitor allows for the
individual assessment of the glucose excursion (GI) of various foods.
What To Eat and What Not to Eat
The most important intervention to reduce obesity, metabolic syndrome, type
II diabetes, cancer, cardiac disease, neurodegenerative diseases, autoimmune
diseases etc., is to eat real food and not processed food. Telling the
difference is quite simple. If it looks like food, it is real. If it comes
in a box or has a food label, it’s likely processed. The more ingredients
listed on a product’s label and the more chemicals you see with strange and
unpronounceable names, the more processing the product has undergone. Recent
evidence suggests that processed foods in themselves can cause insulin
resistance.
Healthy foods include:
-
All vegetables, especially avocados, and cruciferous and leafy
vegetables.
-
Fish (wild caught fresh fish especially Alaskan/Pacific salmon and
sardines)
-
Eggs (they’ve been giving a bad rap!); free range “organic” eggs are
suggested.
- Chicken breast (free range, no hormones, no antibiotics)
-
Nuts especially almonds, brazil nuts, cashews, and pistachios.
-
Unsweetened Peanut butter (but avoid the white bread and grape jelly!)
and Chia seeds
- Meat (grass-fed, no hormones, avoid processed meats)
- Blueberries (limit volume if insulin resistant)
-
Coffee, with heavy cream or coconut oil; choose Stevia (without
erythritol) over sugar or artificial sweeteners.
Flattening the Glucose Curve
Apart from carbohydrate restriction/ketogenic diet and time-restricted
eating, several simple interventions (or hacks) prevent the high glucose
spikes that fuel cancer. The book “Glucose Revolution” by Jessie Inchauspe
is highly recommended and provides more details on interventions to flatten
the blood glucose curve, such as.
Eat Foods in the Right Order
Veggies (greens/fiber) should be eaten first, protein and fat second, and
starch (sugars) last; this slows gastric emptying, as well as the breakdown
and absorption of glucose. Eat fruit last; always preceded by fiber. Don’t
begin a meal with bread (starch).
-
Begin all meals with a salad or green vegetables. Use olive oil and
vinegar as salad dressing.
- Avoid starchy foods with no fiber.
-
Avoid fruit juices and smoothies, which cause a large glucose spike.
-
Skip breakfast. Breakfast is the worst time to eat sugar and starches;
this results in a large glucose spike. Cereal for breakfast causes a rapid
spike in glucose.
- Avoid snacking throughout the day.
-
Drink a tablespoon of vinegar stirred into a tall glass of water before
eating starch or something sweet. Apple cider vinegar is recommended. The
acetic acid in vinegar decreases the enzymatic breakdown of starch,
increases glycogen synthesis (and glucose uptake), and increases fatty
acid oxidation. Vinegar may be beneficial even if consumed up to 20
minutes after a starchy food. Apple cider vinegar is usually unpasteurized
and should be avoided in pregnancy.
-
If vinegar is not readily available, consume a few fiber tablets (esp.
glucomannan tablets) before eating a starchy/sweet treat.
-
Go for a 20-minute walk within an hour of eating/having starchy food.
During exercise, muscles take up glucose for energy while increasing
mitochondrial oxidative capacity. Going to the gym or doing resistance
exercise is an alternative. Climbing a few stairs is an option at work. If
sedentary, do sitting calf raises (the soleal pump). The soleal pump is
strongly recommended; it has been demonstrated to reduce postprandial
glucose by about 50%, reduce hyperinsulinemia, and improved lipid
metabolism. When engaging in fasted exercise (before eating), the liver
releases glucose into the bloodstream to fuel the mitochondria in the
muscles causing a glucose spike. This is mediated by an increased release
of cortisol, epinephrine, and norepinephrine (with decreased glucagon);
i.e., release of harmful stress hormones. If exercising before eating, we
suggest instead of a regular protein shake, consider a shake with
‘superfoods,’ e.g., Ka’Chava ™ or 310 Shakes.™ Shakes should include
ingredients like plant protein, a super green, omega-3 fatty acids,
vitamins, adaptogenic herbs, probiotics, fiber, super mushrooms, and
berries.
Establishing/Restoring a “Normal” Microbiome
The microbiome has a remarkable effect on blood sugar levels and insulin
sensitivity. (372-378) Establishing a normal microbiome is important for
regulating blood glucose levels and improving insulin sensitivity.
Furthermore, alterations in the microbiome play an important role in both
tumorigenesis and tumor propagation. Follow these suggestions to help
establish a “normal microbiome”:
- Eat a diverse range of foods.
-
Eat lots of vegetables, legumes, and beans. • Eat fermented foods like
yogurt (unsweetened), kefir, apple cider vinegar, kombucha, pickles,
sauerkraut, tempeh, and kimchi.
-
Eat foods rich in polyphenols (dark fruits). Include resveratrol
supplements.
-
Eat prebiotic fiber. Glucomannan is a dietary fiber (soluble and
insoluble) made from the root of the konjac plant.
- Eat chia seeds, high in insoluble and soluble fiber.
- Eat less sugar and sweeteners.
- Reduce stress.
- Avoid taking antibiotics unnecessarily.
- Stop snacking.
- Exercise regularly.
-
Spend time outdoors in the natural world to get exposure to millions of
microbes, many of which can benefit microbiome diversity.
- Get enough sleep.
The consumption of fermented foods may be particularly important in
restoring/maintaining a normal microbiome. Large cohort studies as well as
limited interventional studies have linked the consumption of fermented
foods with weight maintenance and decreased diabetes, cancer, and
cardiovascular disease risks.
The Saturated Fat-Cholesterol Hoax
The Cholesterol-Saturated fatty acid hoax began to proliferate in the 1960s.
Dr. Ancel Keys popularized the notion that saturated fats and high
cholesterol were the primary causes of atherosclerotic heart disease — the
so-called Diet-Heart Hypothesis. This concept has been vigorously studied,
including in many RCTs, and has been convincingly proven to be false. (357,
384, 385) Indeed, replacing saturated fats with a diet high in vegetable
oils (linoleic acid) was associated with higher rates of death,
cardiovascular and coronary heart disease as well as a significantly
increased risk of cancer. (386)
Healthy and Unhealthy Oils Avoid seed oils high in linoleic acid. Linoleic
acid is an Omega-6 fatty acid that our bodies require in small amounts.
Unfortunately, many people eat up to 10 times the desired amount of linoleic
acid, because of excess consumption of foods made with seed oils. Too much
linoleic acid is associated with inflammation, obesity, heart disease, and
other unfavorable conditions. Therefore, avoid the following oils:
Soybean oil, Corn oil, Cottonseed oil, Sunflower oil, Sesame oil, Grapeseed
oil, Safflower oil, Rice bran oil, Margarine.
Instead, opt for healthy oils and fats such as the ones listed below. Use
only high-quality products and check production and expiration dates.
-
Olive oil (oleic acid, omega-9 monounsaturated fatty acids); never heat
olive oil to the point where it produces smoke.
-
Avocado oil (oleic acid, omega-9 monounsaturated fatty acids)
- Coconut oil (medium chain fatty acid)
- Flaxseed oil (alpha-linolenic acid, ALA omega-3)
-
Walnut and pecan oils; should be refrigerated to avoid spoilage
- Butter (saturated fat)
Breast cancer women with lifestyle intervention 56% less likely to have died
from breast cancer compared to women in the control group
A landmark prospective, randomized study evaluated the short- and long-term
effects of a comprehensive lifestyle intervention on women with stage II or
stage II breast cancer who had undergone surgery. (
ACS Journal 2008)
The intervention included techniques to reduce stress, improve “quality of
life,” and promote healthy behaviors including guidance on diet, exercise,
relaxation techniques, social support and healthy living.
Patients in the intervention group attended regular sessions with follow-up
appointments to ensure compliance with the lifestyle program. Eleven years
later, women who participated in the intervention had a 45% lower risk of
cancer recurrence than those in the control group and were 56% less likely
to have died from breast cancer compared to women in the control group.
Intervention patients were also 49% less likely than women in the control
group to die from any cause.
The study also demonstrated that women who took part in the intervention had
significantly improved psychological, behavioral, and health outcomes, as
well as improved immune function compared with patients in the control
group.
Best Cancer Fighting Supplements: Evidence Based
What vitamins minerals etc can help fight cancer?
We have ranked the top cancer fighting supplements
to help you with your research. We have also
organised and summarised relevant and salient
research information in one place. Below, we look
at the most published and studied categories.
Important Note: This
information is for educational purposes only and
should not be interpreted as medical advice.
Here is the list (listed in order of importance):








.png)

.png)




.png)

Comments
Post a Comment