Paxlovid vs Molnupiravir: Which is Better? (2024)
What is Paxlovid?
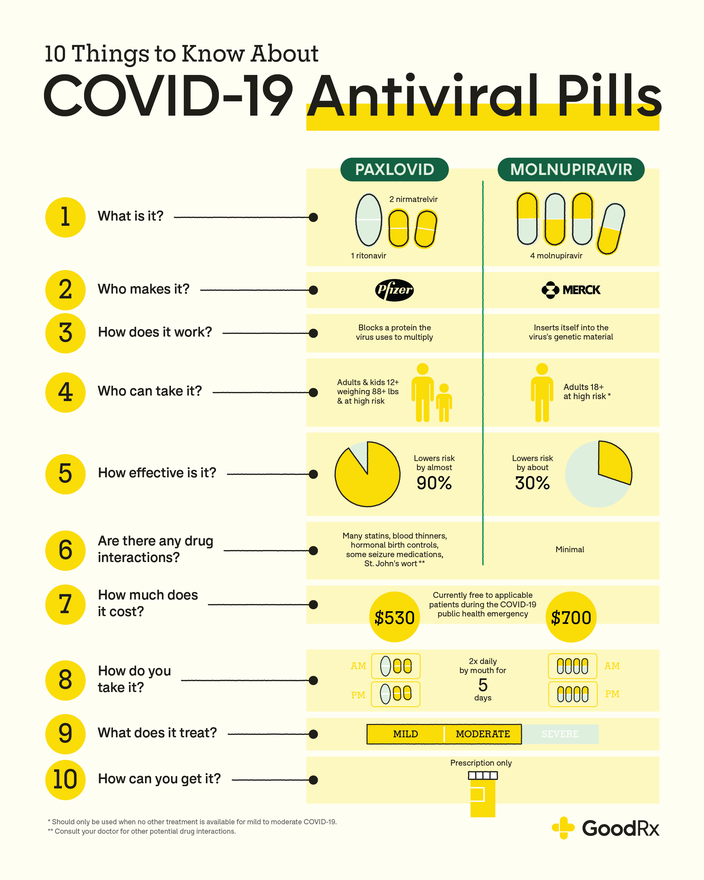
Paxlovid is a combination of two antiviral pills: nirmatrelvir and ritonavir.Update: The Latest Research About Paxlovid: Effectiveness, Access, and Possible Long COVID Benefits (JAMA Sep 2024)
How does Paxlovid work for COVID-19?
The two medications in Paxlovid work together to help treat COVID-19. Both nirmatrelvir and ritonavir belong to the same class of medications: protease inhibitors.
Nirmatrelvir stops the virus that causes COVID-19 from copying itself. The virus relies on an enzyme (protein) in our bodies called protease to copy itself. Nirmatrelvir temporarily stops this enzyme from working so the virus can’t use it to multiply.
Ritonavir helps slow the breakdown of nirmatrelvir. This helps nirmatrelvir stay in the body at higher levels for longer. In other words, ritonavir helps make nirmatrelvir more effective against COVID-19 than it would be on its own.
What is molnupiravir?
Molnupiravir is also an oral antiviral pill authorized to treat mild to moderate COVID-19. This medication, manufactured by Merck, received EUA shortly after Paxlovid. Molnupiravir is authorized for adults ages 18 and older that are at high risk of severe COVID-19. However, the FDA has stated it should only be used if no other recommended COVID-19 treatments are available.
Update: Health outcomes 3 months and 6 months after molnupiravir treatment for COVID-19 for people at higher risk in the community (PANORAMIC): a randomised controlled trial (The Lancet Infectious Diseases Sep 2024)How does molnupiravir work for COVID-19?
Molnupiravir is a nucleoside analog. It also stops the COVID-19 virus from copying itself. But it does this in a different way than Paxlovid.
Molnupiravir looks like genetic building blocks that the COVID-19 virus uses to copy itself. So when you take the medication, the virus mistakenly inserts molnupiravir into its genetic material. When this happens, the virus can’t copy itself.
How are Paxlovid and molnupiravir dosed and given?
Paxlovid and molnupiravir have several similarities when it comes to taking each medication. The biggest dose difference lies in how many pills you take at one time.
Paxlovid comes as a prepackaged carton containing 30 tablets. For each dose, you’ll take two nirmatrelvir tablets and one ritonavir tablet. These three pills should be taken by mouth twice daily for 5 days. Swallow the pills whole. Don’t split, chew, or crush them.
A molnupiravir prescription comes with 40 capsules. Take four capsules by mouth twice daily (every 12 hours) for 5 days. The capsules should be swallowed whole. Don’t open or crush the capsules.
Paxlovid and molnupiravir should be started within 5 days of when a person first starts experiencing COVID-19 symptoms. Both COVID-19 pills can be taken with or without food. It’s also important to finish all the medication prescribed to you to help them be as effective as possible.
How effective are Paxlovid and molnupiravir for treating COVID-19?
The effectiveness is probably the most notable difference between Paxlovid and molnupiravir.
In clinical trials, Paxlovid was nearly 90% effective at preventing hospital stays or death due to COVID-19 in high-risk people. Ongoing clinical trials suggest Paxlovid is about 70% effective in people with a standard risk of severe illness.
On the other hand, molnupiravir lowered the risk of COVID-19 hospital stays or death by about 30% in high-risk people. This difference in effectiveness may be one of the reasons the FDA suggests using molnupiravir only if other treatments aren’t available.
It’s important to note that these levels of effectiveness were recorded when study participants started Paxlovid or molnupiravir within 5 days of first feeling symptoms. The medications’ effectiveness is lower if the medications are started after this timeframe.Paxlovid for long COVID
The complex medical condition involves more than 200 symptoms ranging from exhaustion and cognitive impairment to pain, fever and heart palpitations that can last for months and even years following a COVID-19 infection.
According to details of the study, posted on Clinicaltrials.gov, the randomized, placebo-controlled trial will test Pfizer's treatment or a placebo in 1,700 volunteers aged 18 and older.
The Duke Clinical Research Institute is supervising the study, which is scheduled to start on Jan. 1, 2023. The trial will investigate a leading theory of the cause of long COVID, which holds that fragments of the virus persist in the tissues of some individuals, causing prolonged symptoms.
Patients in several case studies have reported improvements in their symptoms after taking Pfizer's antiviral treatment, and several physicians have called for the drug to be studied in a large, scientifically rigorous study in patients with long COVID.
Paxlovid, which combines a new Pfizer pill with the old antiviral ritonavir, is currently authorized for use in the first days of a COVID infection to prevent severe disease in high-risk patients.
Estimates of long COVID prevalence range from 5 to 50% of people who have had a COVID-19 infection. It affects people who have had both mild and severe COVID-19, including children, and can be severe enough to keep people out of work.
Paxlovid Rebound
At the same time, the CDC wrote, “a brief return of symptoms may be part of the natural history of [coronavirus] infection in some persons, independent of treatment with Paxlovid and regardless of vaccination status.”
The CDC said there is no evidence for more treatment in rebound cases, though people should isolate again for at least 5 days so they won’t pass COVID-19 to others.
People who have a rebound after taking Paxlovid can report their cases to Pfizer’s adverse event reporting page.
Researchers found that when patients received a placebo instead of treatment, a portion of them still experienced a rebound of their symptoms after they had initially improved.
“Symptom return is common,” said Dr. Davey Smith, the chief of infectious diseases and global public health at the University of California, San Diego School of Medicine, who led the study. “It doesn’t mean that things are going south. It’s just the natural way the disease goes.” What is surprising, however, is how many people may experience a rebound, he said. (Read More)
“I think when you're using drugs such as Paxlovid for only 5 days with one specific protease inhibitor, the nirmatrelvir component of the drug, it may be that that is not sufficient.”
What are the known side effects of Paxlovid and molnupiravir?
Side effects for both Paxlovid and molnupiravir were mild for most people in clinical trials.
Common Paxlovid side effects include:
-
Changes in taste
-
Diarrhea
-
High blood pressure
-
Muscle aches
Molnupiravir side effects reported most often include diarrhea, nausea, and dizziness.
What are the serious side effects of Paxlovid and molnupiravir?
As with any medication, Paxlovid and molnupiravir have risks of more serious side effects. Each medication’s risks are unique. These could be a reason why a healthcare provider may pick one COVID-19 pill over the other.
Paxlovid can be hard on both the liver and kidneys. It’s possible this medication could damage these organs, especially if you already have issues with them. Paxlovid isn’t recommended if you have liver problems. If you have kidney problems, you may need a different dose of Paxlovid. Depending on your personal risks, your healthcare provider may also choose to avoid prescribing it.
Protease inhibitors are often used to treat HIV. As mentioned above, Paxlovid contains two protease inhibitors. This medication may cause HIV to become resistant to other HIV medications if you aren’t being fully treated for the condition. If you have HIV, discuss whether Paxlovid is the best choice for you with your healthcare provider.
Molnupiravir shouldn’t be taken if you're pregnant. Animal studies suggest that molnupiravir may harm unborn babies or cause a miscarriage. If you are sexually active and able to get pregnant, you should use reliable birth control while taking molnupiravir and for 4 days after your last dose.
There’s also concern that molnupiravir may affect sperm. Experts are unsure if this could affect a future pregnancy. As an extra precaution, it’s recommended for males who are sexually active with a person who can get pregnant to use condoms while taking molnupiravir. They should also continue using condoms for at least 3 months after their last dose.
Molnupiravir also shouldn’t be taken by people under 18 years old. This is because the medication may affect bone and cartilage development in younger people. Discuss the best COVID-19 treatment option for your child or teen with their healthcare provider.
What interactions do Paxlovid and molnupiravir have?
Paxlovid interacts with many medications. Some interactions make Paxlovid less effective, and others make it too plentiful in your body.
The following list includes some of the most notable interactions. But there are many other medications that may be unsafe to combine with Paxlovid. Discuss all medications and over-the-counter (OTC) products you take with your healthcare provider and pharmacist.
According to FDA's Fact Sheet for Healthcare Providers (Section 5.1):
5.1. Risk of Serious Adverse Reactions Due to Drug Interactions- Clinically significant adverse reactions, potentially leading to severe, life-threatening, or fatal events from greater exposures of concomitant medications.
- Clinically significant adverse reactions from greater exposures of PAXLOVID.
- Loss of therapeutic effect of PAXLOVID and possible development of viral resistance.
Some of Paxlovid’s interactions include:
-
Certain medications that treat heart rhythm problems, such as amiodarone (Pacerone)
-
Certain medications used to control seizures, such as carbamazepine (Tegretol)
-
Certain statin cholesterol medications, such as simvastatin (Zocor)
-
Colchicine (Colcrys)
-
Ergot medications, such as dihydroergotamine mesylate (D.H.E. 45, Migranal)
-
Forms of birth control that include an estrogen called ethinyl estradiol
-
Lurasidone (Latuda)
-
Sildenafil (Revatio) when used to treat pulmonary arterial hypertension (PAH)
-
St. John’s wort — an OTC herbal product

|
Currently, molnupiravir isn’t known to interact with any medications. This is still being studied and may change as more information becomes available. Always discuss all your medications with your healthcare provider before starting a new medication.
How much do Paxlovid and molnupiravir cost?
Definite cost information for Paxlovid and molnupiravir still isn’t available. The original prices reported were around $530 for a course of Paxlovid and about $700 for a round of molnupiravir.
More recently, news reports have stated that Paxlovid will be provided to all U.S. states at no cost. Merck has previously stated the cost of molnupiravir will also be lower than the original price given. But an exact cost hasn’t been confirmed for either.
We’ll know more about their costs as these COVID-19 pills become available. Quantities of Paxlovid and molnupiravir are limited at this time. More courses of the two antiviral treatments will be shipped to the U.S. during 2022.Can you take Paxlovid and molnupiravir together for COVID-19?
No. This combination hasn’t been studied for any use, including COVID-19. An interaction between Paxlovid and molnupiravir isn’t listed by either Pfizer or Merck. But due to the lack of research about whether this is safe or effective, this combination isn’t suggested.Efficacy of Paxlovid and Molnupiravir against Omicron Subvariants BQ.1.1 and XBB | NEJM (Dec 2022)
The NIH COVID-19 Treatment Guidelines Panel’s Statement on Therapies for High-Risk, Non-hospitalized Patients With Mild to Moderate COVID-19 (Last Updated: April 20, 2023)
Preferred therapies. Listed in order of preference:
- Ritonavir-boosted nirmatrelvir (Paxlovid)
- Remdesivir
- Molnupiravir
Alternative therapies for outpatients with COVID-19
The bottom line
Paxlovid and molnupiravir are the first two oral medications available for treating mild or moderate COVID-19. Both medications are authorized for high-risk people. They should be started within 5 days of first feeling symptoms.There are several similarities between Paxlovid and molnupiravir. But the biggest difference lies in how effective they’ve been for COVID-19 in studies. If you’ve recently tested positive for COVID-19, talk to your healthcare provider to see if either COVID-19 pill is an option for you.
Sources and References
Paxlovid vs. Molnupiravir (Lagevrio) for COVID-19: goodrx.comAssociated Press. (2021). Merck asks US FDA to authorize promising anti-COVID pill.
Centers for Disease Control and Prevention. (2021).
COVID-19 information for specific groups of people.
Centers for Disease Control and Prevention.
(2021). People with certain medical conditions.
Centers for Disease Control and Prevention. (2022). COVID data tracker.
Food and Drug Administration. (2021). Coronavirus (COVID-19) update: FDA authorizes additional oral antiviral for treatment of COVID-19 in certain adults.
Food and Drug Administration. (2021). Emergency use authorization 105.
Food and Drug Administration. (2021). Emergency use authorization 108.
Food and Drug Administration. (2021). Fact sheet for healthcare providers: emergency use authorization for molnupiravir.
Food and Drug Administration. (2021). Fact sheet for healthcare providers: emergency use authorization for Paxlovid.
HIVinfo. (2022). Protease inhibitor (PI).
Merck & Co., Inc. (2021). Fact sheet for healthcare providers: emergency use authorization for molnupiravir.
Merck Sharp & Dohme Corp. (2021). Merck and Ridgeback Biotherapeutics provide update on results from MOVe-OUT study of molnupiravir, an investigational oral antiviral medicine, in at risk adults with mild-to-moderate COVID-19.
Nature. (2021). How antiviral pill molnupiravir shot ahead in the COVID drug hunt.
NBC News. (2021). FDA authorizes first Covid pill, from Pfizer, for emergency use.
Pfizer Inc. (2021). Pfizer announces additional phase 2/3 study results confirming robust efficacy of novel COVID-19 oral antiviral treatment candidate in reducing risk of hospitalization or death.
Pfizer Inc. (2021). Pfizer’s novel COVID-19 oral antiviral treatment candidate reduced risk of hospitalization or death by 89% in interim analysis of phase 2/3 EPIC-HR study.
Pfizer Inc. (2021). Pfizer receives U.S. FDA emergency use authorization for novel COVID-19 oral antiviral treatment.Pfizer Inc. (2021). Protease inhibitors to fight COVID-19: stopping the virus’s life cycle.
Reuters. (2021). U.S. to buy 10 mln courses of Pfizer's COVID-19 pill for $5.3 bln.

.png)
.png)






Comments
Post a Comment