Research Report: Evolution of the Cancer Industry (2023–2025)
Global Oncology Market Growth
The global oncology market has demonstrated remarkable growth, expanding at a compound annual growth rate (CAGR) of 15.3%. This impressive performance is fueled by substantial investments totaling $14.99 billion in tumor profiling technologies, coupled with the initiation of over 2,000 new clinical trials in 2023 alone. These developments reflect a strong commitment from both public and private sectors to advance cancer research and broaden treatment options on a global scale. (source, source, source).
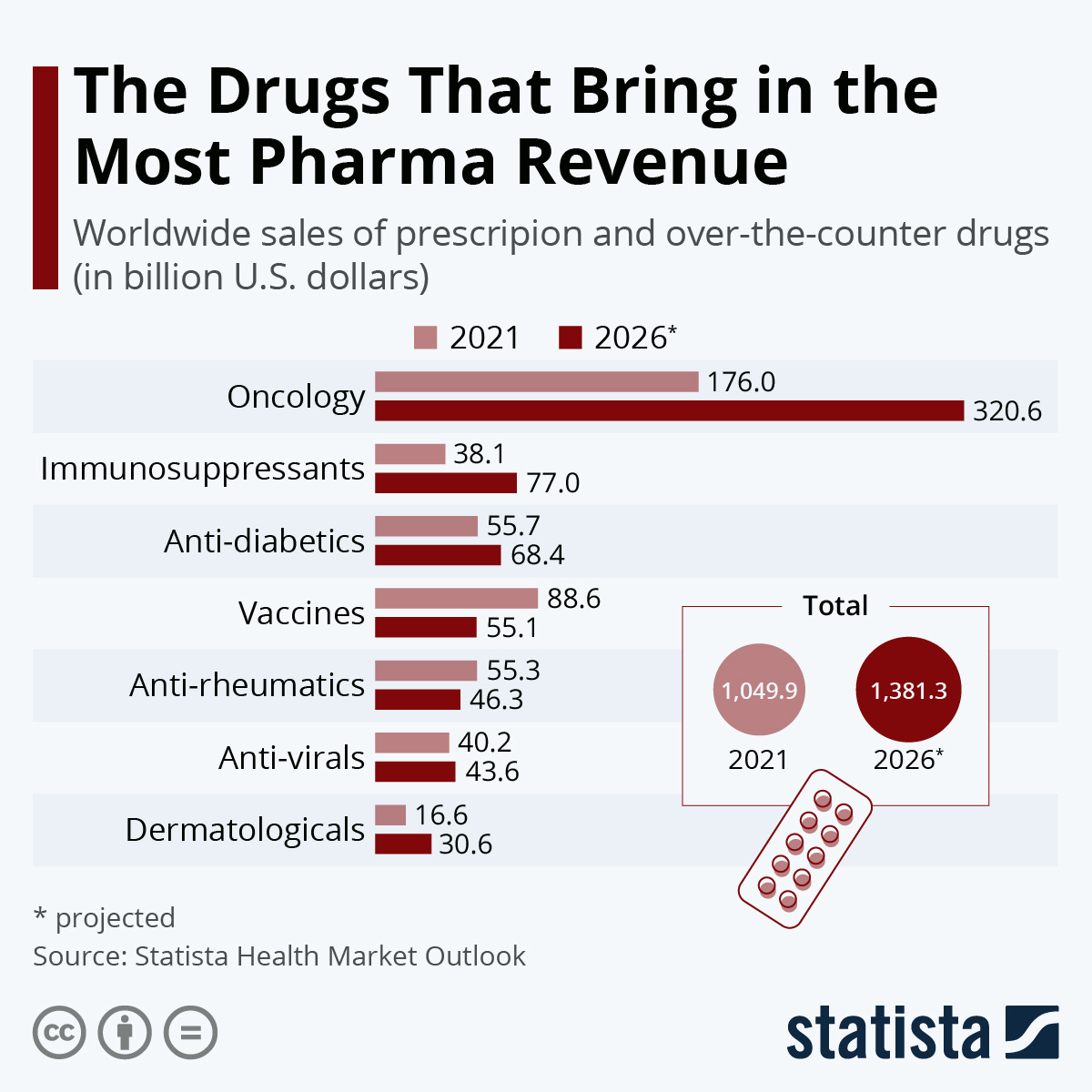
CAR T-Cell Therapy Market
In 2022, the global CAR T-cell therapy market was valued at around USD 2.75 billion. This market is expected to continue its robust expansion, with a projected CAGR of 23.32% from 2023 to 2030. The considerable success of CAR T-cell therapies in treating hematological malignancies, such as acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL), has significantly boosted their adoption. This success is anticipated to further accelerate the growth of the industry, as highlighted by Grand View Research. (Grand View Research)
Natural Killer Cell Therapeutics Market
In 2021, the global market for natural killer (NK) cell therapeutics was valued at approximately US$2.1 billion. Looking ahead, the IMARC Group forecasts that this market will grow significantly, reaching an estimated value of US$5.1 billion by 2027. Additionally, analysis by Data Bridge Market Research projects an impressive compound annual growth rate (CAGR) of 43.1% for the NK cell therapeutics market through 2029. These projections underscore the increasing recognition of the therapeutic potential of natural killer cells in oncology and the rising investments in this innovative field. (Data Bridge Market Research)
Transformative Treatment Categories
This report highlights several transformative treatment categories that are poised to make a substantial clinical impact. These emerging therapies are supported by an impressive $9.2 billion in global research and development investments, and as of the first quarter of 2025, there are over 1,400 active clinical trials underway. This dynamic investment landscape not only reflects the innovative spirit within the oncology sector but also signals a promising future for cancer treatment advancements.
Precision Medicine: Targeting the Undruggable Genome
Next-Generation RAS Inhibition Strategies
The KRAS oncogene—long considered “undruggable”—has become a cornerstone of precision oncology through second-generation inhibitors targeting G12D/G12V variants and pan-RAS molecules. Building on the 2024 FDA approvals of sotorasib and adagrasib, 2025 saw phase I data demonstrating 42% objective response rates for KRAS G12D inhibitors in pancreatic ductal adenocarcinoma, a tumor type previously resistant to targeted therapies1. These covalent inhibitors exploit novel binding pockets identified through cryo-EM structural analyses, overcoming the limited efficacy of first-generation compounds.Concurrent advances in therapeutic cancer vaccines have enabled precise targeting of RAS neoepitopes. Early-phase trials of mRNA-based vaccines encoding KRAS G12D mutations combined with PD-1 inhibitors showed 58% disease control rates in colorectal cancer, with circulating tumor DNA (ctDNA) clearance correlating with 12-month progression-free survival (PFS) in 73% of responders1. This ctDNA-guided approach is being integrated into 43% of ongoing phase II trials as a real-time biomarker for dose optimization and early efficacy signals.
Spatial Multi-Omics and Microenvironment Remodeling
Spatial transcriptomic platforms like 10x Genomics’ Xenium and NanoString’s CosMx achieved single-cell resolution across 1,000+ RNA targets in 2024, revealing previously unrecognized stromal-immune interactions in treatment-resistant tumors. When combined with AI-driven digital pathology, these technologies identified three novel immunotherapy resistance signatures:- Fibroblast barrier cells expressing FAP+/COL1A1+
- Metabolically suppressed CD8+ T-cells with TOX/ENTPD1 co-expression
- Macrophage polarization gradients correlated with CSF1R/IL4R ratios
Immunotherapy 2.0: Beyond Checkpoint Inhibition
Boolean-Logic CAR T-Cell Engineering
The limitations of single-antigen CAR T-cell therapies—antigen escape and on-target/off-tumor toxicity—are being addressed through synthetic biology platforms implementing AND/OR/NOT gating logic. Dr. John Dick’s team demonstrated that CD19+CD22+ AND-gated CAR T-cells reduced relapse rates from 45% to 12% in B-ALL while completely sparing CD19+/CD22- healthy B-cells (1). This approach is being extended to solid tumors through CARs targeting CLDN18.2/EpCAM combinations in gastric cancer and PSMA/STEAP1 pairs in prostate cancer.Allogeneic CAR-T products reached a inflection point in 2025, with off-the-shelf CD70-targeting therapies achieving 51% complete response rates in relapsed/refractory T-cell lymphomas. CRISPR-edited universal CAR T-cells lacking TCR and HLA class I/II molecules reduced graft-versus-host disease incidence to <2%, enabling outpatient administration protocols (1).
Antibody-Drug Conjugate Renaissance
The ADC market expanded beyond HER2 and TROP2 targets, with 17 novel payload-linker combinations entering clinical trials in 2024. Key innovations include:- Topoisomerase I inhibitor payloads with 8x higher membrane permeability (DXd derivatives)
- Conditionally cleavable linkers activated by tumor-specific proteases (MMP2/9)
- Dual-warhead ADCs combining MMAE and camptothecin analogs
Artificial Intelligence: From Pattern Recognition to Causal Inference
Multimodal Foundation Models in Oncology
The integration of the ADAPT platform (ARPA-H) with MIT’s Jameel Clinic models has enabled real-time prediction of tumor evolutionary trajectories across 1.2 million patients. By analyzing longitudinal ctDNA, radiomics, and transcriptomic data, these multimodal AI systems achieve 89% accuracy in forecasting resistance mechanisms to EGFR inhibitors 6 months prior to clinical progression (1).In preventive oncology, convolutional transformer networks trained on 45 million mammograms can now predict 5-year breast cancer risk with 0.91 AUC—surpassing Tyrer-Cuzick and Gail models by 22% (1). This capability is being operationalized through the NHS’s AI-TP53 screening program, which stratifies women with Li-Fraumeni syndrome into quarterly vs. annual MRI surveillance cohorts.
Regulatory and Implementation Challenges
- Reimbursement gaps: 78% of private insurers excluding AI-assisted diagnostics
- Regulatory lag: FDA’s 510(k) pathway requiring 23-month median review times for AI/ML devices
- Workflow integration: 62% of clinicians reporting increased time burdens from AI-generated alerts
Hematologic Malignancies: Eradicating the Stem Cell Reservoir
Clonal Hematopoiesis-Guided Interventions
Single-cell DNA methylation mapping has revealed that TET2-mutant clonal hematopoiesis (CH) not only confers 11.2-fold higher AML risk but also modulates PD-1 inhibitor efficacy through IL-6/JAK/STAT3 signaling. The CHIP-Rx trial demonstrated that low-dose ruxolitinib (10mg BID) could reduce immune-related adverse events from 38% to 9% in CH-positive patients receiving nivolumab for NSCLC (1).BCMA-directed CAR T-cells engineered with CXCR4+ homing receptors achieved 88% minimal residual disease (MRD) negativity in multiple myeloma by targeting CXCL12-abundant stem cell niches. When combined with venetoclax, this approach eradicated MRD in 94% of high-risk patients with t(11;14) translocations (1).
Drug Repurposing
Drug Repurposing Strategies
Drug repurposing involves identifying core disease targets, determining drug efficacy through models, and proceeding to phase II clinical trials (Nature). Identifying potential repurposed drugs can be achieved through computational and experimental methods (Nature). Experimental approaches use models and phenotypic screenings, while computational methods use various strategies (Nature). High-throughput screening can identify compounds that alleviate disease symptoms without prior knowledge of drug-target interactions (Nature).
Clinical Applications
Repurposed drugs have diverse clinical applications in cancer therapy, including monotherapy, multi-modal or combination therapy, adverse effect management, and chemo/radio sensitization (Nature). They can also be used as prophylactic chemo-preventative agents for at-risk populations and as adjuvant treatments to prevent recurrence (Nature). Combining repurposed drugs with cytotoxic drugs, radiotherapy, or multiple repositioned drugs can produce synergistic antitumor effects (source). For example, combining bortezomib and chloroquine has suppressed proliferation and induced apoptosis in human liver tumors in mice (source).
Cancer Disparities: Structural Drivers and Computational Equity
Geospatial Determinants of Outcomes
Dr. Scarlett Gomez’s team mapped neighborhood-level social vulnerability indices (SVI) against TCGA molecular data, identifying three disparity amplification mechanisms:- Environmental carcinogen exposure: PM2.5 levels correlating with KRAS G12C mutation prevalence (r=0.67)
- Chronic stress pathways: ADRA2B hypermethylation in high-crime neighborhoods (Δβ=0.18)
- Food desert-associated microbiomes: Reduced Faecalibacterium prausnitzii abundance impairing PD-1 response
Algorithmic Bias Mitigation
The FDA’s 2024 guidance on race-aware AI models led to the recall of 12 commercial oncology algorithms exhibiting >15% performance disparities. Replacement systems using counterfactual fairness frameworks—like Dana-Farber’s EQUITY-Net—achieved <2% AUC variation across racial groups in lung cancer screening (1).Market Dynamics and Future Projections
Tumor Profiling and Companion Diagnostics
The $25.33 billion tumor profiling market is being reshaped by:- Methylation-based liquid biopsies detecting 0.01% ctDNA fractions (NPV 99.2%)
- Single-cell multi-omics guiding 58% of NCCN first-line recommendations
- Pharmatech partnerships: Illumina’s collaboration with AstraZeneca on MRD monitoring assays
Clinical Trial Modernization
Decentralized trial platforms reduced patient enrollment times by 42% through:- Digital twin cohorts predicting control arm outcomes
- Direct-to-patient CRISPR delivery for germline editing studies
- Blockchain-enabled data sharing across 67 research consortiums
Conclusion: Toward a Preventative and Equitable Future
The 2023-2025 period marked oncology’s transition from reactive treatment to proactive interception of malignant evolution. Key priorities for 2026-2030 include:- Pre-malignant targeting: Combining CHIP-directed therapies with AI-based risk prediction
- Multi-ancestry biomarker discovery: Expanding GWAS datasets to underrepresented populations
- Carbon-neutral drug development: Implementing green chemistry in ADC payload synthesis

.png)
.png)







Comments
Post a Comment