Bebtelovimab vs Paxlovid: What's the Difference?
Most people with COVID-19 will experience a mild illness, and they’ll be able to take care of themselves at home. But some—especially those with underlying health conditions—could benefit from one of several COVID-19 treatments. Some of these are available in pill form and others are given intravenously or by injection—and all of them must be prescribed by a health care provider.
BA.5 became the dominant subvariant in the US earlier this month, surpassing BA.2.12.1. The BA.4 Omicron subvariant is the second most prevalent with 12.8% of cases originating from the pathogen, while the BA.2.12.1 subvariant now accounts for only 8.6%.
BA.5 is one of many Covid-19 Omicron subvariants to emerge since last winter. The subvariant is also driving up cases in parts of Europe and North America and has become the dominant U.S. Omicron strain. This version of the virus is believed to spread particularly easily, fueled in part by its ability to evade immunity built up from vaccines and prior infections.
Only a handful of scientific studies have been published on BA.4 and BA.5 so far, meaning knowledge about them remains limited.
In this article, we will do a roundup and cover 2 popular anti-viral
treatments i.e. Paxlovid and Bebtelovimab, a Monoclonal Antibody treatment.
Paxlovid
How to Get Paxlovid Without a Medical Doctor
In March 2022, the Biden administration launched the Test to Treat initiative. The initiative’s goal was to allow people to be tested for COVID-19 at
pharmacies or health centers that have an on-site clinic, like CVS
MinuteClinic. If you had a positive test, you’d then receive an antiviral
prescription at the pharmacy before leaving the building.
While
well-intentioned, there were many roadblocks that made the Test to Treat
initiative difficult to roll out. One big barrier was the fact that most
pharmacies don’t have clinics in the same building. In late May 2022,
there were only about 2,500 Test to Treat locations in the entire U.S. This left many Americans still struggling to get
a prescription from their healthcare providers in enough time to benefit
from Paxlovid.
In response to this, the FDA updated Paxlovid’s
EUA in July 2022. This change allows pharmacists to prescribe Paxlovid directly to people who have tested positive for COVID-19. This
means that you might be able to head over to your local pharmacy for the
medication without having to make other stops along the way. Keep in mind
that not all pharmacies may offer this service.
- Your current health records, which must be less than a year old (either electronic or printed records are fine)
- Your most recent liver and kidney function tests, which must be less than a year old
- A complete list of all medications that you take, including any over-the-counter (OTC) medications, vitamins, and supplements
How much will Paxlovid cost?
New Paxlovid Dose Pack Authorized by FDA
- The standard packaging that is currently in distribution: 300 mg nirmatrelvir;100 mg ritonavir - Each carton contains 30 tablets divided in 5 daily-dose blister cards. Each blister card contains 4 nirmatrelvir tablets (150 mg each) and 2 ritonavir tablets (100 mg each). Nirmatrelvir tablets and ritonavir tablets are supplied in separate blister cavities within the same child-resistant blister card.
- The new packaging option that will be in distribution later this month: 150 mg nirmatrelvir; 100 mg ritonavir - Each carton contains 20 tablets divided in 5 daily-dose blister cards. Each blister card contains 2 nirmatrelvir tablets (150 mg each) and 2 ritonavir tablets (100 mg each). Nirmatrelvir tablets and ritonavir tablets are supplied in separate blister cavities within the same child-resistant blister card.
Paxlovid Rebound
At the same time, the CDC wrote, “a brief return of symptoms may be part of the natural history of [coronavirus] infection in some persons, independent of treatment with Paxlovid and regardless of vaccination status.”
The CDC said there is no evidence for more treatment in rebound cases, though people should isolate again for at least 5 days so they won’t pass COVID-19 to others.
People who have a rebound after taking Paxlovid can report their cases to Pfizer’s adverse event reporting page.
Bebtelovimab (Monoclonal Antibody)
Bebtelovimab is an US FDA-authorized investigational monoclonal antibody treatment that was developed by Eli Lilly. Not all authorized monoclonal antibodies have worked against all of the SARS-CoV-2 variants. However, data showing bebtelovimab’s efficacy against Omicron and its BA.2 subvariant prompted the FDA to authorize the drug through an EUA. It is meant for people who have a current COVID-19 infection.

|
| Credit: Design Cells/SPL |
When it was authorized: February 2022.
Who can get it: Adults and children ages 12 and up who weigh at least 88 pounds. They must have a positive COVID-19 test result and be at high risk for developing severe COVID-19.
How you take it: An intravenous injection is given for at least 30 seconds. Patients are observed by a health care provider for at least an hour after injection. Bebtelovimab must be given within seven days of symptom onset.
Side effects: There is limited information known about the safety and effectiveness of bebtelovimab for the treatment of mild-to-moderate COVID-19, according to the FDA fact sheet. The sheet also provides a list of potential side effects the FDA recommends reporting to a medical provider, and reports that allergic reactions can happen during and after injection. Because bebtelovimab is still being studied, it’s possible that all of the risks aren’t yet known.
How it works: It binds to the spike protein that causes COVID-19, similar to other monoclonal antibodies that have shown efficacy against hospitalization and death from the disease.
How well it works: The EUA for bebtelovimab was supported by clinical and nonclinical data that showed it has efficacy against Omicron and its BA.2 subvariant. The clinical data was based on a Phase 2 trial that treated non-hospitalized patients with bebtelovimab alone or together with another drug called etesevimab. That study is available in a preprint, which has not yet been peer-reviewed.
What else you should know: There is limited experience treating pregnant women or breastfeeding mothers. So, those patients should discuss their options and specific situation with their health care provider.
The NIH considers this to be an alternative treatment, which should be used only when neither of the NIH-preferred therapies (Paxlovid and remdesivir) are available, feasible to use, or clinically appropriate.
More information: FDA bebtelovimab fact sheet for patients, parents, and caregivers.
The COVID-19 Treatment Guidelines Panel’s Statement on Therapies for High-Risk, Non-hospitalized Patients With Mild to Moderate COVID-19 (Last Updated: August 8, 2022)
Preferred therapies. Listed in order of preference:
- Ritonavir-boosted nirmatrelvir (Paxlovid)
- Remdesivir
- Bebtelovimab
- Molnupiravir
Effectiveness of Monoclonal Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants | NEJM
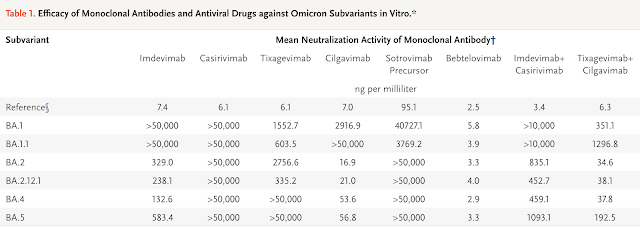

Effectiveness of Monoclonal Antibodies and Antiviral Agents against the Omicron Subvariant BA.2.75
REGN10987 (marketed as imdevimab) lost neutralizing activity against BA.2.75, whereas REGN10933 (marketed as casirivimab) retained some neutralizing activity against the isolate. REGN10987 in combination with REGN10933 (casirivimab–imdevimab) inhibited BA.2.75; however, the neutralizing activity against BA.2.75 with this combination was less than that against the ancestral strain (SARS-CoV-2/UT-NC002-1T/Human/2020/Tokyo) by a factor of 812.5.


.png)

.png)






Comments
Post a Comment