Inferior Curcumin and Turmeric products sold on Amazon: Tests find potency, labeling, heavy metals and other problems

Photo © AdobeStock.com/Victor Moussa
In June 2021, dietary supplements company NOW (Bloomingdale, IL) purchased from Amazon.com 23 non-NOW turmeric/curcumin supplement products, plus two of its own supplement products, and tested them for potency, heavy metals, labeling accuracy, and potential addition of synthetic curcumin. NOW describes the test results of non-NOW brands as “poor.”
Most products passed initial potency tests. Of those that performed poorly in potency tests, one product failed and four others tested low-potency, but none were advertised with a specific label claim.
Common label claim problems were discovered, however. Many products were mislabeled to infer that there was more curcumin in the product than was actually present. For instance, a product’s front panel might be labeled as “turmeric curcumin 1650 mg,” but in fact the ingredient listing on the side panel revealed that the 1650 mg represented not just the total amount of turmeric/curcumin in the product but also other ingredients present—for instance, turmeric root (1500 mg), ginger root (300 mg), turmeric extract (150 mg), and BioPerine piperine (15 mg) per three capsules. “That equals to 655 mg per capsule, and less than 10% of the product is turmeric 95% standardized extract,” NOW points out.
“This can be perceived as deceptive since many customers do not know the difference between turmeric, turmeric extract, curcumin extract, and standardized 95% extract,” said Dan Richard, NOW’s vice president of global sales and marketing, in a press release.
Potency testing was done both at NOW’s internal labs and at Eurofins labs. The assay method used was RP-HPLC with UV detection, and potencies were determined based on total curcuminoids per capsule.
When it came to heavy metals like arsenic, cadmium, lead, and mercury, the average total heavy metals present in the non-NOW products was 525% higher than the two samples of NOW’s products. In fact, only one non-NOW product out of 23 was found to contain fewer heavy metals than NOW’s products. Specifically, NOW points to two brands, B’Leaf Nature and Eagle brands, whose products contained heavy metal levels more than 20 times higher than NOW’s and above California’s Prop 65 limits for lead. Two others, from brands Farm Haven and BioEmblem, both measured above 100 ppb in cadmium, which NOW says is a particularly toxic heavy metal.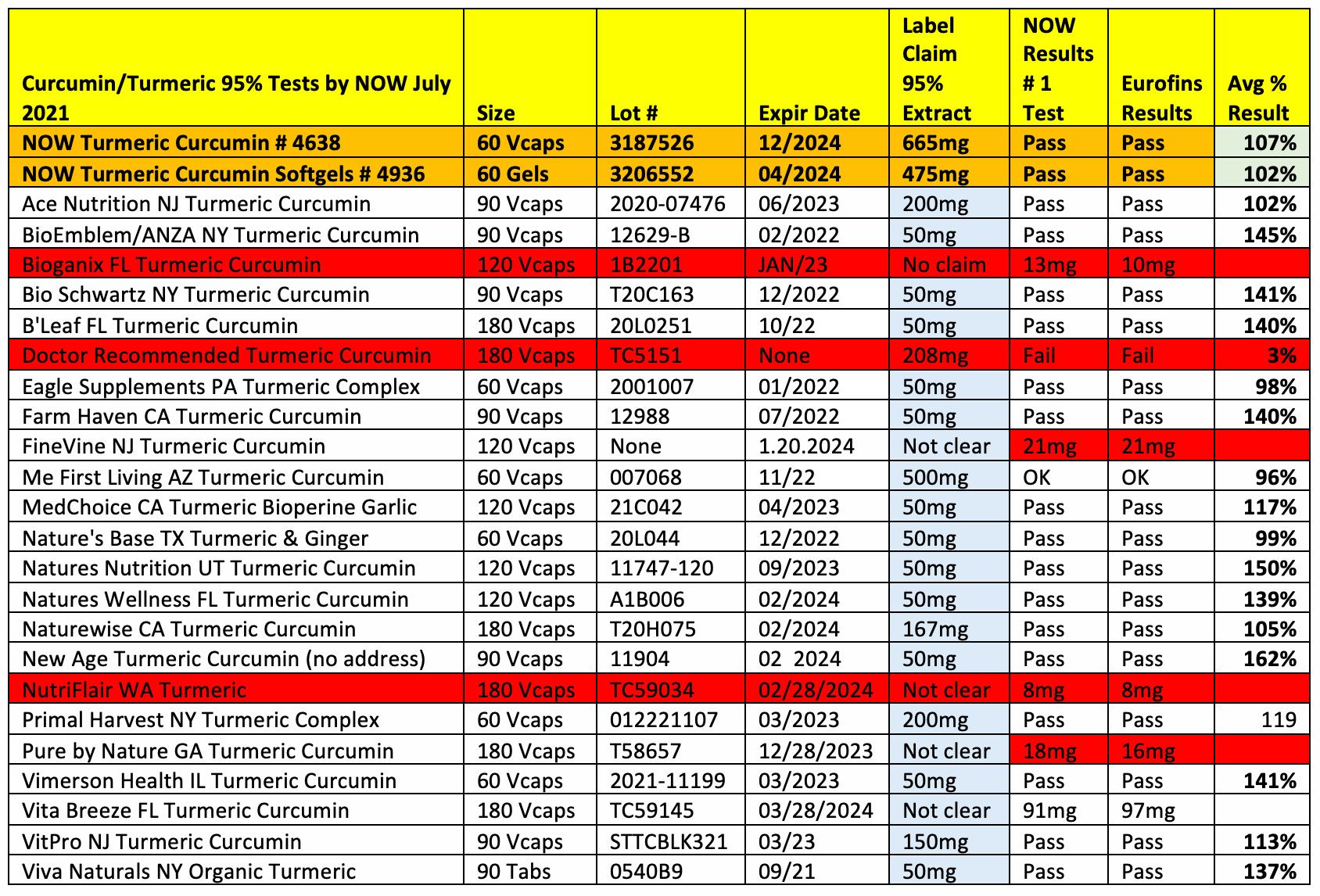
Synthetic adulteration is also a growing concern in the turmeric/curcumin market and thus was also tested in NOW’s analysis. Synthetic curcumin is derived from petrochemicals and is much cheaper because it doesn’t use a natural source of turmeric. NOW says it sent all samples to the University of Georgia’s Center for Applied Isotope Studies for independent radiocarbon testing. The lab found that 4 out of 23 non-NOW brands—Vitpro, Me First Living, Eagle, and Primal Harvest—were spiked with “fossil fuel–derived organic carbon.”
Vegetarian labeling was also misleading. NOW found that 2 of the 23 samples (from Bioganix and Nutriflair) were labeled as vegetarian when in fact they contained animal-derived gelatin capsules.
In total, 12 out of 23 non-NOW products tested failed for potency, containing synthetic curcuminoids, heavy metals, or use of gelatin caps instead of the claimed veggie caps. On a positive note, 11 of the 23 passed all tests, though with slightly misleading labeling, the company says. Furthermore, NOW says it was the only brand to list both total and net weights of 95% extract on labels. For instance, NOW’s product #4638 is labeled as 665 mg turmeric extract providing 630 mg or 95% curcumin as the primary active potency.
NOW has tested other supplement categories sold on Amazon in the past, including alpha lipoic acid (ALA) and CoQ10 and SAMe. The company says it is part of its ongoing effort “to disclose alarming quality failings and misleading, inaccurate labels among unfamiliar brands of products purchased on Amazon.”
“While we appreciate Amazon’s initial efforts to address these ongoing, egregious problems with sellers on their platform, there is clearly still a long way to go,” Richard said. “The kind of results we found are not what consumers expect when they purchase dietary supplements from sellers they trust.”
Related: Curcumin and COVID-19 Studies
.png)
.png)







Comments
Post a Comment