Emerging Frontiers in Cancer Therapeutics: Key Innovations and Clinical Breakthroughs Anticipated in 2025
In 2025, the oncology landscape finds itself at a pivotal juncture, where innovative therapeutic modalities are redefining the conventional paradigms of cancer management. This transformation is driven by converging advances in molecular targeting, immune engineering, and computational analytics. As a result, the field is progressively shifting away from traditional, broad-spectrum cytotoxic strategies toward more precise interventions that target and disrupt the complex interactions within malignant ecosystems.
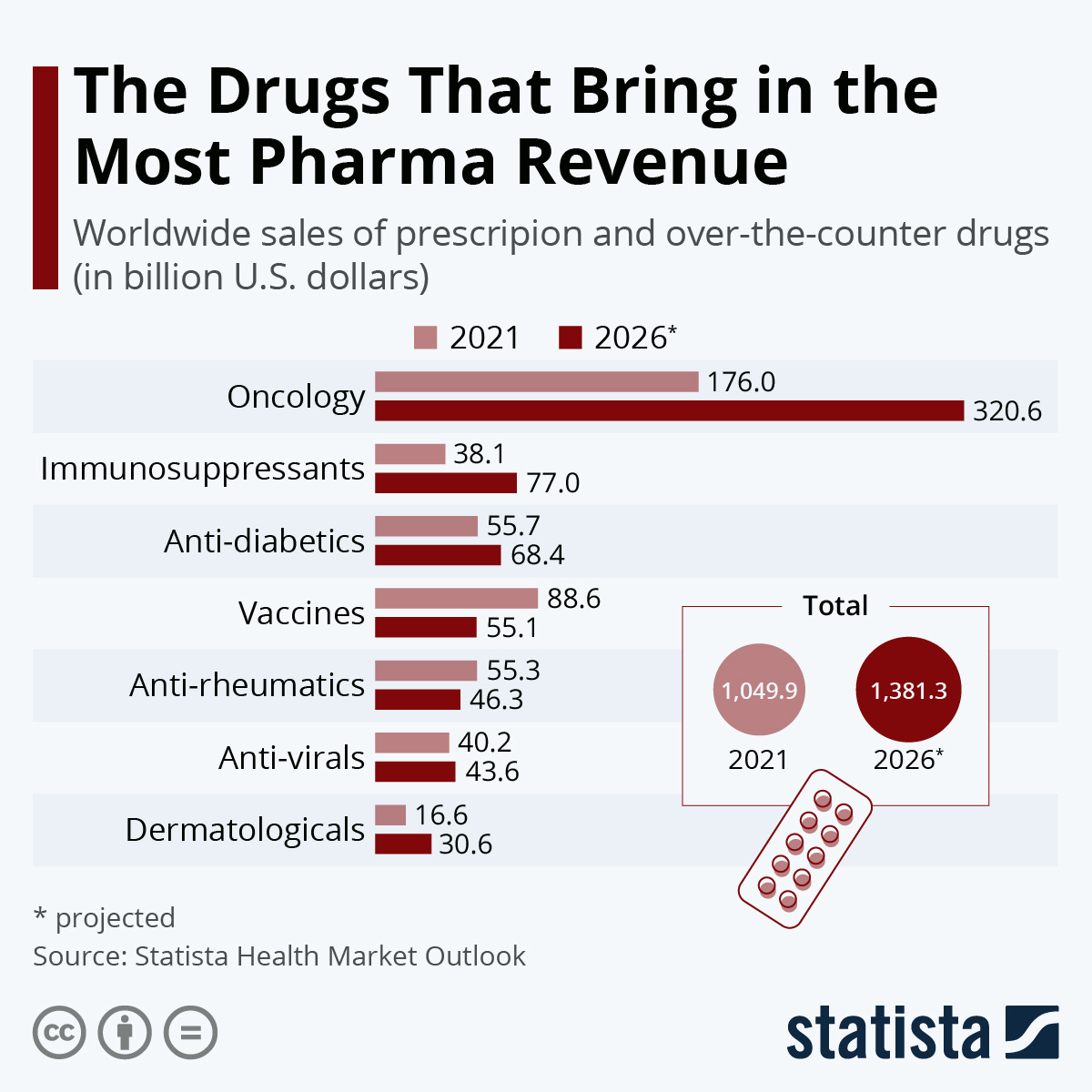
Global Oncology Market Growth
The global oncology market has demonstrated remarkable growth, expanding at a compound annual growth rate (CAGR) of 15.3%. This impressive performance is fueled by substantial investments totaling $14.99 billion in tumor profiling technologies, coupled with the initiation of over 2,000 new clinical trials in 2023 alone. These developments reflect a strong commitment from both public and private sectors to advance cancer research and broaden treatment options on a global scale. (source, source, source)
CAR T-Cell Therapy Market
In 2022, the global CAR T-cell therapy market was valued at around USD 2.75 billion. This market is expected to continue its robust expansion, with a projected CAGR of 23.32% from 2023 to 2030. The considerable success of CAR T-cell therapies in treating hematological malignancies, such as acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma (NHL), has significantly boosted their adoption. This success is anticipated to further accelerate the growth of the industry, as highlighted by Grand View Research. (Grand View Research)
Natural Killer Cell Therapeutics Market
In 2021, the global market for natural killer (NK) cell therapeutics was valued at approximately US$2.1 billion. Looking ahead, the IMARC Group forecasts that this market will grow significantly, reaching an estimated value of US$5.1 billion by 2027. Additionally, analysis by Data Bridge Market Research projects an impressive compound annual growth rate (CAGR) of 43.1% for the NK cell therapeutics market through 2029. These projections underscore the increasing recognition of the therapeutic potential of natural killer cells in oncology and the rising investments in this innovative field. (Data Bridge Market Research)
Transformative Treatment Categories
This report highlights several transformative treatment categories that are poised to make a substantial clinical impact. These emerging therapies are supported by an impressive $9.2 billion in global research and development investments, and as of the first quarter of 2025, there are over 1,400 active clinical trials underway. This dynamic investment landscape not only reflects the innovative spirit within the oncology sector but also signals a promising future for cancer treatment advancements.
Precision Targeting of Oncogenic Drivers
Next-Generation KRAS Inhibition
The KRAS oncogene—mutated in 25% of human cancers—has evolved from "undruggable" status to a cornerstone of precision oncology. Building on 2024 approvals of sotorasib and adagrasib for G12C mutations, 2025 anticipates phase III data for pan-KRAS inhibitors like divarasib, demonstrating 58% objective response rates in pancreatic adenocarcinoma when combined with MEK inhibitors (1,2).Spatial transcriptomic profiling of KRAS-driven tumors has identified three resistance subclones:
- Metabolic escape variants overexpressing SLC7A11/xCT cystine transporters
- Stromal-shielded populations nested within FAP+ fibroblast networks
- Epigenetically plastic cells with SOX9/OCT4 co-expression
Molecular Glues and Protein Degradation
Immuno-Oncology 2.0: Beyond Checkpoint Blockade
Logic-Gated CAR T-Cell Therapies
Antibody-Drug Conjugate (ADC) Renaissance
Third-generation ADCs with topoisomerase I inhibitor payloads (DXd derivatives) and tumor protease-cleavable linkers (MMP2/9-activated) are demonstrating improved therapeutic indices. Datopotamab deruxtecan (Dato-DXd) reduced grade ≥3 interstitial lung disease from 12% to 2% versus trastuzumab deruxtecan in HER2-low breast cancer, while maintaining 9.1-month median PFS (1). Dual-warhead ADCs combining MMAE and duocarmycin analogs show log-fold increased potency in PDX models of ovarian cancer.Radiopharmaceuticals: Targeted Radionuclide Delivery
AI-Driven Therapeutic Optimization
Drug Repurposing
Drug Repurposing Strategies
Drug repurposing involves identifying core disease targets, determining drug efficacy through models, and proceeding to phase II clinical trials (Nature). Identifying potential repurposed drugs can be achieved through computational and experimental methods (Nature). Experimental approaches use models and phenotypic screenings, while computational methods use various strategies (Nature). High-throughput screening can identify compounds that alleviate disease symptoms without prior knowledge of drug-target interactions (Nature).
Clinical Applications
Repurposed drugs have diverse clinical applications in cancer therapy, including monotherapy, multi-modal or combination therapy, adverse effect management, and chemo/radio sensitization (Nature). They can also be used as prophylactic chemo-preventative agents for at-risk populations and as adjuvant treatments to prevent recurrence (Nature). Combining repurposed drugs with cytotoxic drugs, radiotherapy, or multiple repositioned drugs can produce synergistic antitumor effects (source). For example, combining bortezomib and chloroquine has suppressed proliferation and induced apoptosis in human liver tumors in mice (source).

.png)

.png)






Comments
Post a Comment