Top 10 drugs by worldwide sales in 2021 (2022 Update)
Three other COVID products catapulted into the top 20 rankings last year. Moderna’s mRNA vaccine Spikevax was the third-highest selling product at $17.7 billion. Meanwhile, Regeneron and Roche's antibody treatment REGEN-COV and Gilead’s infused antiviral Veklury finished in the No. 13 and No. 19 slots, respectively, both generating more than $5 billion.
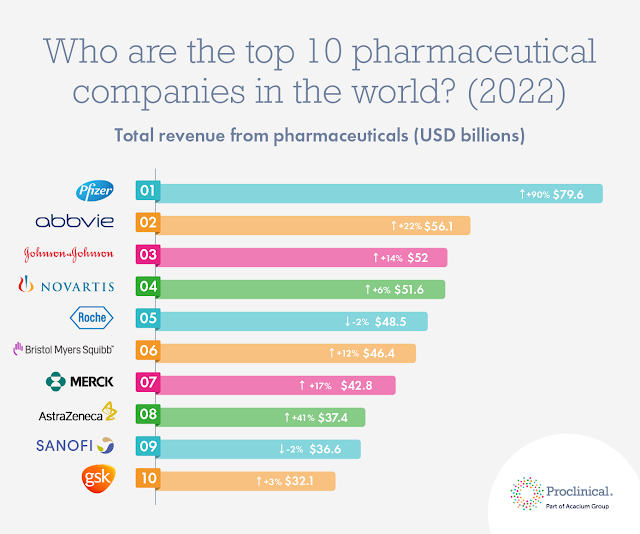 |
| Credit: ProClinical.com |
In a boom year for the industry, the No. 20 medicine on the list, Pfizer’s breast cancer drug Ibrance, generated sales of $5.4 billion. It was quite a departure from 2020 when the No. 20 drug on the list, Johnson & Johnson’s Remicade, pulled in $4.2 billion.
Big sales of the COVID meds brought more volatility to the top 20 list than usual with seven new products. From 2018 to 2019, there were four new drugs in the top 20. The list saw the same turnover from 2019 to 2020.
Of the seven products that dropped off the list from 2020, the only one that saw a significant increase in sales was AstraZeneca’s lung cancer drug Tagrisso, which went from $4.3 billion in 2020 to $5 billion last year. Roche’s Rituxan, J&J’s Remicade and Amgen’s Enbrel all dropped out after more than a decade in the top 20. Also falling out were Pfizer’s Prevnar family of vaccines, Pfizer and Astellas’ cancer drug Xtandi and Roche’s multiple sclerosis med Ocrevus.
Figures for this report come courtesy of Drug Discovery & Development, which compiles a list annually of the world's top 50 drugs, and company filings. Currency conversions were made in mid-May 2022.
1. Comirnaty
Companies: Pfizer, BioNTech2021 sales: $36.8 billion
Disease: COVID-19
From a revenue perspective, no pharmaceutical product has had a better year than Pfizer and BioNTech’s Comirnaty did in 2021. First to authorization in the U.S. and first to a full approval, the partners’ mRNA-based COVID-19 shot has eclipsed AbbVie's Humira as the world’s best-selling drug—and it’s poised for an encore in 2022. Still, the future of COVID-19 vaccine sales—not just for Pfizer but for all COVID-19 players—looks increasingly murky. Comirnaty remains destined for tens of billions in sales this year, to be sure, but an ongoing debate over the need for boosters, plus uncertainty about the shift from a pandemic market to a private one, suggests COVID-19 may not line Pfizer’s coffers forever.
Comirnaty generated a staggering $36.8 billion, with Pfizer recording all of its sales and then paying royalties to partner BioNTech, which rang up 19 billion euros ($19.89 billion) from the vaccine.
For 2022, Pfizer expects to reap $32 billion in Comirnaty sales. BioNTech, for its part, is expecting between 13 billion euros ($13.68 billion) and 17 billion euros ($17.89 billion).
Pfizer and BioNTech’s vaccine scored a full approval back in August, which immediately boosted confidence in the shot among Americans, a Harris Poll survey found at the time. Before that, the shot scored its first FDA emergency use authorization in December 2020.
In a bid to reach as many people as possible, Comirnaty got a major boost in October when the FDA endorsed it for use in kids ages 5 to 11. That decision immediately unlocked the door to a population of some 28 million children in the U.S.
Meanwhile, the booster debate, which has poured into 2022, started raging back in September 2021 when the FDA declined to authorize an initial booster dose for all patients. At the time, the regulator signed off on a third Comirnaty dose for older Americans and those with risk factors such as diabetes and obesity.
Pfizer and BioNTech scored their general booster nod in January, opening up a third dose for patients ages 12 and up.
Looking ahead, the COVID-19 vaccine market could eventually go the way of influenza, with people needing annual vaccinations, many experts have suggested. Pfizer has hinted at its Comirnaty plans for the private market, but, so far, nothing material has taken shape.
Still, Pfizer scientists think it’s “unlikely that [SARS-CoV-2] will be fully eradicated in the foreseeable future,” CEO Albert Bourla, Ph.D., said on a February earnings call.
And, while COVID-19 vaccine demand appears to be slumping now, there’s potential for a rebound in the second half of the year, analysts have suggested.
“It does look like the phasing of vaccine sales this year could be back-end loaded,” Cantor Fitzgerald analyst Louise Chen wrote in a recent note to clients, adding that she believed Pfizer was “best positioned” to adapt to new strains of the virus and future recommendations that may result.
2. Humira
2021 sales: $20.7 billion
Diseases: Rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, plaque psoriasis and more
AbbVie’s immunology superstar Humira has long held the drug sales crown, but the times in biopharma are changing. Thanks to high pandemic demand, Pfizer’s megablockbuster COVID-19 vaccine knocked Humira off the No. 1 position last year. And while Humira still turned in an impressive $20.7 billion worldwide, copycat competition is here to eat away at the key brand.
Note: HUMIRA (Adalimumab) is an injectable TNF blocker medicine that can lower the ability of your immune system to fight infections. It can be used alone, with methotrexate, or with certain other medicines.
European biosimilars launched in 2018 and have been gaining steam since then, causing Humira’s international sales to decline by nearly 10% in 2021 to $3.36 billion. In the U.S., Humira’s days of turning in sales growth are numbered. Last year, Humira delivered $17.3 billion in U.S. sales, a 7.6% increase from 2020.
Starting early next year and throughout 2023, Humira will face a growing number of U.S. biosimilar rivals. Under AbbVie’s settlements with biosimilar players, the rivals will launch in a staggered manner throughout the year, starting with Amgen in late January. The high number of biosim players eager to launch means AbbVie will likely lose more market share than if just one or two copycat launches were nearing the market.
Under that expected pressure, AbbVie is turning its immunology focus to Skyrizi and Rinvoq to help fill the gap once Humira rolls over the patent cliff. The company has been racking up new uses for the pair of drugs and expects them to deliver a combined $15 billion in global sales by 2023.
AbbVie's efforts to grow and defend its brand haven't been without controversy. The company has been accused of building a "patent wall" around the drug. It also aggressively raised prices over the years, according to a 2021 congressional report.
Humira debuted in 2002 and has FDA approvals to treat 10 diseases including rheumatoid arthritis, plaque psoriasis, Crohn's disease and psoriatic arthritis.Related: Natural Rheumatoid Arthritis Treatment and Sabin Protocol
3. Spikevax
Company: Moderna2021 sales: $17.7 billion
Disease: COVID-19
In the business of COVID-19 vaccines, second place is a major win. That's especially true for a company with no other major revenue sources.
That was the story last year for Moderna, when Spikevax reaped $17.7 billion and drove the mRNA specialist into biopharma stardom.
Heading into the pandemic, Moderna was little known outside of the biopharma world. But early into the crisis, the company and federal researchers leaped toward the front of the pack in the COVID-19 vaccine development race.
After quickly pushing through clinical studies, the team won an FDA authorization in December 2020. Since then, Moderna has worked to generate additional data, advance other mRNA vaccine candidates and scale up capacity.
In all, its manufacturing efforts led to deliveries of 807 million doses worldwide last year. And that meant big sales for the budding company.
For the first time last year, Moderna joined the list of top 20 pharma companies by sales. Its $18.5 billion in global revenues represented more than a twentyfold increase from the mRNA specialist's 2020 sales.
The company has had to swiftly add to its ranks to support the growth. As of the end of 2019, the company employed 830 people. By the end of 2021, the number had grown to 2,700.
While Moderna’s vaccine is approved by the FDA in people 18 and older, the company is seeking an authorization for its use in children as young as 6 months old.
After Moderna’s shot generated $17.7 billion in 2021, the company projects $21 billion in sales this year based on orders already signed. In recent months, COVID-19 vaccine makers have said they expect sales to be backloaded in 2022.4. Keytruda
Company: Merck & Co.2021 sales: $17.2 billion
Diseases: Melanoma, non-small cell lung cancer, head and neck cancer, Hodgkin lymphoma, urothelial carcinoma, gastric cancer and more.
As expected, Merck & Co.’s Keytruda was the world’s best-selling cancer drug—again—in 2021. With a $17.19 billion showing and 19.5% year-over-year growth, Keytruda's haul came in more than $4 billion ahead of Bristol Myers Squibb’s Revlimid, the second-ranking oncology asset on our list.
And, compared with Keytruda's closest in-class rival, sales for the Merck drug nearly doubled revenues for Bristol Myers Squibb's Opdivo.
Having already established an unshakeable market lead in metastatic non-small cell lung cancer, Keytruda has been branching out into new indications lately. In 2021, the PD-1 inhibitor snagged FDA approvals for use alongside Roche’s Herceptin in HER2-positive gastric cancer and in combination with Eisai-partnered Lenvima in newly diagnosed, advanced kidney cancer.
Advancing into early cancer treatment is considered the next major growth avenue for PD-1/L1 inhibitors. About half of Keytruda’s growth through 2025 will come from early-stage treatment around surgery, reaching roughly 30% of total sales in 2025, Merck’s newly minted CEO Rob Davis said during a conference call in February.In early-stage disease, Keytruda in 2021 got an FDA go-ahead in a regimen for high-risk, early-stage, triple-negative breast cancer both before and after surgery, and it also became available for certain kidney disease patients following surgery.
In a phase 3 trial, Keytruda recently topped placebo at keeping disease from returning and keeping patients alive when used after surgery to treat stages 1B to 3A non-small cell lung cancer. Merck touted a benefit in a broader patient population regardless of their PD-L1 expression status, which could give it an advantage over Roche’s Tecentriq.
Meanwhile, Keytruda's large pool of positive data across various cancer types has made it the checkpoint inhibitor partner of choice for potential combinations. Besides Lenvima and AstraZeneca-partnered Lynparza, Merck has also been pairing Keytruda with antibody-drug conjugates. Most notably, Astellas and Seagen are expecting potential registrational data soon for their Padcev with Keytruda in first-line bladder cancer.
Related: Expert Explains Cancer May Be Metabolic Disease, and Shares a Cure
5. Eliquis
Companies: Bristol Myers Squibb, Pfizer2021 sales: $16.73 billion
Diseases: Nonvalvular atrial fibrillation, deep vein thrombosis and pulmonary embolism
Since its debut in early 2013, blood thinner Eliquis has been a reliable growth driver for Bristol Myers Squibb and Pfizer. And, while the drug's days of growth clearly aren’t over, Bristol Myers Squibb is already preparing for its eventual downfall.
Partners BMS and Pfizer teamed up on Eliquis way back in 2007, when the drug was still known by its generic name of apixaban. Under their collaboration, the companies jointly develop and commercialize the drug, which BMS originally discovered. Pfizer covers 50% to 60% of development costs, and the companies share global profits and losses equally. In some markets, Pfizer sells the drug and pays BMS a fee.
Under any metric, the partnership has been a success. As Eliquis has scooped up market share from warfarin, its sales have climbed into the top five of pharma products worldwide.
After an initial approval in late 2012 to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, Eliquis nabbed a label expansion in 2014 to treat deep vein thrombosis and pulmonary embolism.
Last year, BMS reported $10.76 billion in Eliquis sales, a 17% increase from 2020. For its part, Pfizer reported 2021 sales of $5.97 billion for the med, a 19% increase. The company said the increase came thanks to continued uptake in nonvalvular atrial fibrilation and market share gains among the class of oral anticoagulants.
But while Pfizer rakes in billions from its successful COVID-19 vaccine and therapeutic, BMS has upcoming patent losses on its mind. The company recently lost exclusivity for its top drug, multiple myeloma therapy Revlimid, and it’s expecting Eliquis to succumb to generics later this decade.
But BMS is not approaching the patent cliff without a plan. Through 2025, the company expects existing drugs, mainly Eliquis and immuno-oncology therapies, to deliver a combined $8 billion to $10 billion in annual growth. Aside from those meds, the company expects new launches to chip in $25 billion of growth or more by 2029.
6. Revlimid
Company: Bristol Myers Squibb2021 sales: $12.8 billion
Diseases: Myelodysplastic syndrome, multiple myeloma, lymphoma, follicular lymphoma
While Revlimid netted BMS $12.8 billion in 2021, the company expects that haul to dwindle to between $9.5 billion and $10 billion in 2022 thanks to a flood of competition in the U.S. and abroad. Each year after, the company said an annual loss of $2 billion to $2.5 billion can be expected.
In the U.S., Israeli-American generics behemoth Teva Pharmaceutical was first to launch its copycat in March 2022.
Teva offers lenalidomide capsules in 5-mg, 10-mg, 15-mg and 25-mg strengths to treat multiple myeloma in combination with dexamethasone, certain myelodysplastic syndromes and mantle cell lymphoma following specific prior treatment. The company didn’t specify the cost for the generic.
Natco Pharma, Sun Pharma, Zydus Cadila, Cipla and Dr. Reddy’s Laboratories are among the companies that have launched U.S. generics or are planning to roll out their copycats. Those companies have struck deals with BMS following patent challenges on Revlimid.
Meanwhile, BMS, knowing Revlimid’s loss will hurt, is eyeing four growth “pillars” to lean on through the end of the decade. Those pillars include immuno-oncology stalwarts Opdivo and Yervoy plus blood thinner Eliquis alongside recent and expected near-term launches, BMS CEO Giovanni Caforio said at this year’s virtual J.P. Morgan Healthcare Conference.
BMS scooped up Revlimid as part of its $74 billion buyout of Celgene in early 2019.7. Biktarvy
Company: Gilead Sciences2022 sales: $10.4 billion
Disease: HIV
HIV drug Biktarvy led the pack, bringing in $10.4 billion over the course of 2022.
If it weren’t for COVID-19, Gilead Sciences’ HIV triplet Biktarvy would still be the world’s best-selling pharmaceutical product for an infectious disease. In 2021, Biktarvy's sales climbed 18.8% year over year to $8.62 billion, single-handedly beating rival GlaxoSmithKline’s entire HIV unit, which brought in 4.78 billion pounds sterling ($5.45 billion).
For Gilead, the strong performance came at a good time. Biktarvy carried Gilead’s flagship HIV franchise during a year when older meds Truvada and Atripla declined dramatically after losing market exclusivity. Biktarvy contains the antiretroviral tenofovir alafenamide, an improved formulation of tenofovir disoproxil fumarate, which are included in the older meds.
Approved by the FDA in 2018, Biktarvy has quickly become the most successful HIV therapy worldwide. Last year, Biktarvy's U.S. market share grew by five percentage points, reaching 42%.
That's the highest level of market share any complete regimen has ever achieved in the U.S., Gilead’s chief commercial officer Johanna Mercier said during a February call with investors.
As of 2021, Biktarvy was the most prescribed HIV treatment for both new and switch patients in the U.S. and the top drug for treatment-naïve patients in five key European markets, Mercier added.
But as GSK notes, its combined dolutegravir-based portfolio, including two-drug regimen Dovato, attracted the most switch patients across the U.S. and EU combined.
Moving into 2022, Biktarvy could see some volume growth in China as it has been included in the country’s National Reimbursement Drug List. That will come at a cost, though, as officials there demand steep price discounts for inclusion in the program.
Meanwhile, the drug faces a threat from long-acting regimens. GSK’s ViiV Healthcare and partner Johnson & Johnson have launched Cabenuva, which can be given every two months, compared with a daily pill like Biktarvy.
Gilead recently settled a high-stakes HIV patent infringement lawsuit with ViiV. It agreed to pay ViiV $1.25 billion plus 3% royalties for future sales of Biktarvy and its bictegravir component in other products in the U.S.
8. Imbruvica
Companies: AbbVie, Johnson & Johnson2021 sales: $9.8 billion
Diseases: Mantle cell lymphoma, chronic lymphocytic leukemia, Waldenstrom’s macroglobulinemia, marginal zone lymphoma, chronic graft-versus-host disease
Generating steadily increasing sales and with the prospect of another decade of exclusivity thanks to AbbVie’s skillful and much scrutinized patenting strategy, Imbruvica has a chance to rise even more among the ranks of the best-selling drugs.
In 2021, AbbVie reported $5.4 billion in Imbruvica sales, a 1.8% increase from its 2020 performance. The company's partner on the med, Johnson & Johnson's Janssen, posted $4.37 billion in worldwide Imbruvica sales last year, a 5.8% increase.
AbbVie picked up its portion of Imbruvica rights in its $21 billion buyout of Pharmacyclics in 2015. Pharmacyclics and J&J's Janssen developed the drug in collaboration and co-market the therapy.
With AbbVie’s megablockbuster immunology drug Humira set to face biosimilars next year, the company is counting on Imbruvica to pick up some of the slack along with fast-rising immunology follow-ons Rinvoq and Skyrizi.
Imbruvica got a key win in federal court last August when it ruled that two makers of generics infringed four of the drug’s patents. AbbVie now doesn’t expect generic competition for Imbruvica until March 2032, the company said last year in a securities filing.
As the first lymphoma drug to hit the market in the BTK class in 2013, Imbruvica was an immediate success and achieved blockbuster status in its second full year on the market. But other companies have followed with their own BTK entries that are starting to pose a threat to Imbruvica’s dominance.
One is AstraZeneca’s Calquence, which won approval in 2017 for mantle cell and then in November of 2019 got a key nod in the much larger chronic lymphocytic leukemia market. Calquence has done well so far, generating (PDF) $1.24 billion in sales last year.
Posing perhaps a greater threat in the more distant future is BeiGene’s Brukinsa. It reached the market six years after Imbruvica but has played catch-up impressively, especially in the clinic.
Brukinsa has won FDA approvals in mantle cell, Waldenstrom’s macroglobulinemia and marginal zone lymphoma. In a head-to-head ALPINE trial against Imbruvica, Brukinsa showed it could trigger a superior response with fewer safety issues in patients with relapsed or refractory CLL/SLL.
9. Stelara
Company: Johnson & Johnson2021 sales: $9.1 billion
Diseases: Plaque psoriasis, psoriatic arthritis, Crohn's disease, ulcerative colitis
Johnson & Johnson’s Stelara entered 2021 still riding the high from 2020, when it grew sales more than 20% despite the pandemic's negative effects.
Launched in 2009, Stelara was the first biologic treatment to selectively inhibit the IL-12 and IL-23 pathways. Since then, it has grown to be J&J's largest product, representing nearly 10% of the healthcare conglomerate's total revenues.
Its patent will expire in September 2023, giving J&J a little more than a year to capitalize before competition eventually comes knocking. In April, Amgen touted a phase 3 win for its biosimilar candidate.
Despite Stelara's demonstrated ability to gain new approvals and wrestle market share, the drug recently failed to outdo AbbVie's megablockbuster Humira in Crohn's disease.
Since Stelara has about one more year of exclusivity, the med may not climb a whole lot higher on the industry's top 20 rankings. But it could turn in a banner year in 2022, and it's not immediately clear when biosimilars will launch in the U.S.
Meanwhile, J&J is putting its marketing muscle behind the drug. The company in April debuted a campaign called "UnstoppaBOLD" that focuses on patient empowerment.
The idea behind J&J's campaign is to show patients “can boldly break through barriers by having an open and honest conversation with their healthcare professional to find a treatment option that works best for them," Tara Muzsi, vice president of sales and marketing, gastroenterology, at J&J biotech unit Janssen, recently said in an interview.10. Eylea
Companies: Regeneron Pharmaceuticals, Bayer2021 sales: $8.9 billion
Diseases: Wet age-related macular degeneration, diabetic macular edema, diabetic retinopathy, macular edema Even as Eylea faces a growing number of competitors, the drug has kept its leadership position in the ophthalmology market.
Eylea is injected into the eye to slow vision loss from diabetic eye disease, wet age-related macular degeneration and other retinal ailments. It blocks VEGF, slowing the growth and leakage of fluid from abnormal blood vessels in the eye.
Regeneron reported $5.79 billion in U.S. sales for the blockbuster eye med last year, a 17% increase from 2020 as demand started to return amid the ongoing pandemic. Bayer, which markets the drug outside the U.S., posted 2.92 billion euros ($3.12 billion), an 18% increase.
A top seller doesn’t come without rivals. After a 2019 FDA approval, market watchers figured Novartis' Beovu could give Eylea's dominance a run. But after Beovu was shown to cause vision-threatening side effects shortly after launch, excitement waned for the newer med. More recently, Roche's Vabysmo also won FDA approvals in wet age-related macular degeneration (AMD) and diabetic macular edema. That drug is expected to bring "fierce competition." In a bid to keep Eylea competitive, Regeneron is advancing a high-dose version that could be given every four months.
Eylea’s patents will start expiring in 2023. Piper Sandler analysts estimate the drug will generate $6.2 billion in U.S. sales this year.
Updated from: https://www.fiercepharma.com/special-reports/top-20-drugs-worldwide-sales-2021
.png)

.png)




.png)

Comments
Post a Comment